高氯酸锂与含氧碳纸的界面效应研究
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
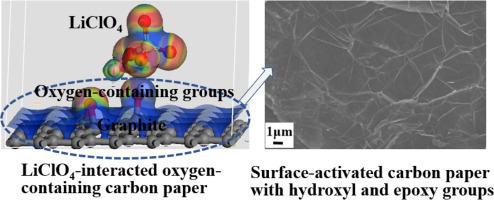
与高氯酸锂相互作用的含氧碳纸(LiClO4-OCP)被设计为具有电活性的超级电容器电极基板,用于储能应用。OCP 是通过在 H2O2 反应介质中对碳纸进行水热活化处理制成的。OCP 由石墨间距组成,表层为超薄石墨烯结构,石墨化程度更高。极化静电力诱导界面吸附的 LiClO4-OCP 比范德华力诱导界面吸附的 LiClO4-CP 显示出更强烈的相互作用,有助于促进 LiClO4-OCP 的界面电荷转移。与 LiClO4-CP 相比,LiClO4-OCP 显示出更有效的界面电荷转移和更可行的电解质扩散,因而具有更高的电化学双层电容。含有含氧基团的 LiClO4-OCP 可进行可逆的氧化还原过程,从而提供额外的法拉第电容。在扫描速率为 5∼100 mV s-1 时,平均响应电流从 LiClO4-CP 的 0.10 ∼ 1.34 mA cm-2 增加到 LiClO4-OCP 的 0.19 ∼ 2.31 mA cm-2,表明 LiClO4-OCP 的电化学活性有所提高。基于循环伏安法的电容从 LiClO4-CP 的 19.91 ∼ 13.01 mF cm-2 mF g-1 增加到 LiClO4-OCP 的 37.76 ∼ 23.06 mF cm-2。电静态充放电电容从 LiClO4-CP 的 13.84 ∼ 3.97 mF cm-2 下降到 LiClO4-OCP 的 29.71 ∼ 12.92 mF cm-2。基于密度泛函理论的模拟计算证明,分子距离如此之短的 LiClO4-OCP 可以发生强烈的静电作用,这种作用是由高氯酸根离子引起的含氧基团极化造成的。与 LiClO4-CP 相比,LiClO4-OCP 具有更低的界面能、更低的带隙和更高的费米能级态密度,这表明 LiClO4-OCP 的界面相互作用和导电性能得到了改善。实验测量和理论计算的结果一致,LiClO4-OCP 电极衬底具有更高的电化学活性,从而显示出其优越的电容性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interfacial effect investigation of lithium perchlorate-interacted oxygen-containing carbon paper
The lithium perchlorate-interacted oxygen-containing carbon paper (LiClO4-OCP) is designed to act as electroactive supercapacitor electrode substrates for the energy storage application. The OCP is fabricated through hydrothermal activation treatment of carbon paper in H2O2 reaction medium. The OCP is composed of graphite pitches with ultra-thin graphene structure of top layer, showing the improved graphitization degree. The LiClO4-OCP with the polarized electrostatic force-induced interfacial adsorption reveals much more intensive interaction than LiClO4-CP with van der Waals force-induced interfacial adsorption, contributing to promoting interfacial charge transfer of LiClO4-OCP. LiClO4-OCP reveals more effective interface charge transfer and more feasible electrolyte diffusion than LiClO4-CP, contributing to higher electrochemical double-layer capacitance. LiClO4-OCP with oxygen-containing groups conducts reversible redox process to supply additional Faradaic capacitance. Mean response current is increased from 0.10 ∼ 1.34 mA cm-2 for LiClO4-CP to 0.19 ∼ 2.31 mA cm-2 for LiClO4-OCP at scan rates of 5∼100 mV s-1, indicating the improved electrochemical activity of LiClO4-OCP. The cyclic voltammetry-based capacitance increases from 19.91 ∼ 13.01 mF cm-2 mF g-1 for LiClO4-CP to 37.76 ∼ 23.06 mF cm-2 for LiClO4-OCP. The galvanostatic charge/discharge-based capacitance decreases from 13.84 ∼ 3.97 mF cm-2 for LiClO4-CP to 29.71 ∼ 12.92 mF cm-2 for LiClO4-OCP. Density-functional theory-based simulation calculation proves LiClO4-OCP with such a short molecular distance is allowed to occur strong electrostatic interaction which is caused by the perchlorate ion-induced polarization of oxygen-containing groups. The LiClO4-OCP has lower interfacial energy, lower band gap and higher density of states at Fermi energy level than LiClO4-CP, indicating the improved interfacial interaction and electrical conductivity of LiClO4-OCP. The experimental measurement and theoretical calculation achieve the consistent results of higher electrochemical activity of LiClO4-OCP electrode substrate to present its superior capacitance performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


