探究厌氧条件下曼氏血吸虫硫氧还蛋白/谷胱甘肽还原酶还原谷胱甘肽的部位
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
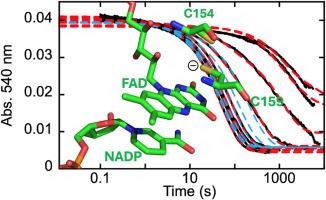
曼氏血吸虫的硫氧还原酶/谷胱甘肽还原酶(SmTGR)是一种多功能酶,可催化谷胱甘肽(GSSG)和硫氧还原酶的还原,以及肽和非肽底物的脱谷胱甘肽化。SmTGR 在结构上类似于已知的谷胱甘肽还原酶(GR)和硫氧还原酶(TrxR),但其 N 端结构域具有典型的谷胱甘肽还原酶(Grx)折叠。尽管与已知的 GR 存在结构同源性,但 GSSG 的还原位点经常被报告为 Grx 结构域,这主要是基于有氧、稳态动力学测量和 X 射线晶体学。在这里,我们对一系列变体 SmTGRs 进行了厌氧表征,以确定 GSSG 还原位点是最靠近 FAD 的半胱氨酸对 Cys154/Cys159,相当于 GRs 中的 GSSG 还原位点。对 U597C、U597S、U597C + C31S 和 I592STOP SmTGR 的厌氧稳态分析表明,Grx 结构域不参与 GSSG 的催化还原,因为 C 端氧化还原沉默不会改变观察到的周转次数(∼0.025 s-1),而 Grx 结构域氧化还原沉默会导致观察到的周转次数增加(∼0.08 s-1)。对这些变体的瞬态单一周转分析证实了这一点,因为观察到的最慢速率随 GSSG 浓度呈双曲线滴定,并接近与每个变体各自的稳态周转次数相吻合的极限。对瞬态数据的数值积分拟合只能在包括 C 端竞争性结合的情况下才能解释观察到的趋势,这表明电子到两种底物的分配发生在 Cys154/Cys159 二硫化物处,而不是之前提出的 Cys596/Sec597 硫化物/硒化物处。矛盾的是,在 Ile592 处截断 C 端会导致 GR 活性丧失,这表明 C 端具有关键的非氧化还原作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Probing the site of glutathione reduction by thioredoxin/glutathione reductase from Schistosoma mansoni under anaerobic conditions
Thioredoxin/glutathione reductase from Schistosoma mansoni (SmTGR) is a multifunctional enzyme that catalyzes the reduction of glutathione (GSSG) and thioredoxin, as well as the deglutathionylation of peptide and non-peptide substrates. SmTGR structurally resembles known glutathione reductases (GR) and thioredoxin reductases (TrxR) but with an appended N-terminal domain that has a typical glutaredoxin (Grx) fold. Despite structural homology with known GRs, the site of GSSG reduction has frequently been reported as the Grx domain, based primarily on aerobic, steady-state kinetic measurements and x-ray crystallography. Here, we present an anaerobic characterization of a series of variant SmTGRs to establish the site of GSSG reduction as the cysteine pair most proximal to the FAD, Cys154/Cys159, equivalent to the site of GSSG reduction in GRs. Anaerobic steady-state analysis of U597C, U597S, U597C + C31S, and I592STOP SmTGR demonstrate that the Grx domain is not involved in the catalytic reduction of GSSG, as redox silencing of the C-terminus results in no modulation of the observed turnover number (∼0.025 s−1) and redox silencing of the Grx domain results in an increased observed turnover number (∼0.08 s−1). Transient-state single turnover analysis of these variants corroborates this, as the slowest rate observed titrates hyperbolically with GSSG concentration and approaches a limit that coincides with the respective steady-state turnover number for each variant. Numerical integration fitting of the transient state data can only account for the observed trends when competitive binding of the C-terminus is included, indicating that the partitioning of electrons to either substrate occurs at the Cys154/Cys159 disulfide rather than the previously proposed Cys596/Sec597 sulfide/selenide. Paradoxically, truncating the C-terminus at Ile592 results in a loss of GR activity, indicating a crucial non-redox role for the C-terminus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


