雄性黑线大鼠肌肉时钟的改变需要限时喂食和限时跑步的协同作用
Q2 Medicine
引用次数: 0
摘要
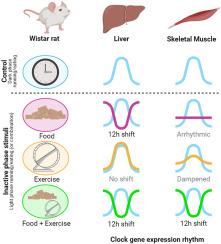
昼夜节律紊乱是导致当今肥胖症和 2 型糖尿病高发的一个重要因素。虽然错误的热量摄入时间对昼夜节律紊乱的影响已得到广泛认可,但错误的体力活动时间对昼夜节律紊乱的影响仍未得到充分研究。在这里,我们通过限制雄性 Wistar 大鼠在先天性非活动(光照)阶段(LR)使用跑步轮,模拟了夜班工人体力活动时间不正确的情况。对照组包括不允许使用车轮(NR);仅在先天活跃期(黑暗期)使用车轮(DR);或不受限制(自由使用)使用车轮(ALR)。LR 不会改变肌肉或肝脏时钟的相位,但会抑制肌肉时钟的振幅。我们之前的研究表明,光相限制进食确实会改变肝脏时钟,但会使肌肉时钟失调,因此我们接下来将轮子和食物的进食时间限制结合到光相(LRLF)或暗相(DRDF)中。LRLF使骨骼肌和肝脏中大多数时钟基因的节律发生了12小时的变化。另一方面,DRDF 在减轻体重和减少脂肪积累方面最为有效。因此,要改变雄性 Wistar 大鼠的肌肉时钟,必须在喂食和体育锻炼的时间上进行协同。这些发现可能有助于进一步改进生活方式策略的设计,从而限制昼夜节律紊乱造成的代谢失调。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergy between time-restricted feeding and time-restricted running is necessary to shift the muscle clock in male wistar rats
Circadian disruption is an important factor driving the current-day high prevalence of obesity and type-2 diabetes. While the impact of incorrect timing of caloric intake on circadian disruption is widely acknowlegded, the contribution of incorrect timing of physical activity remains relatively understudied. Here, we modeled the incorrect timing of physical activity in nightshift workers in male Wistar rats, by restricting running wheel access to the innate inactive (light) phase (LR). Controls included no wheel access (NR); access only during the innate active (dark) period (DR); or unrestricted (ad libitum) access (ALR). LR did not shift the phase of the muscle or liver clock, but dampened the muscle clock amplitude. As our previous study demonstrated that light-phase restricted feeding did shift the liver clock, but made the muscle clock arrhythmic, we next combined the time restriction of wheel and food access to either the light phase (LRLF) or dark phase (DRDF). LRLF produced a ∼12 h shift in the majority of clock gene rhythms in both skeletal muscle and liver. On the other hand, DRDF was most effective in reducing body weight and the accumulation of fat mass. Therefore, in order to shift the muscle clock in male Wistar rats, synergy between the timing of feeding and physical activity is necessary. These findings may contribute to further improve the design of lifestyle strategies that try to limit metabolic misalignment caused by circadian disruption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurobiology of Sleep and Circadian Rhythms
Neuroscience-Behavioral Neuroscience
CiteScore
4.50
自引率
0.00%
发文量
9
审稿时长
69 days
期刊介绍:
Neurobiology of Sleep and Circadian Rhythms is a multidisciplinary journal for the publication of original research and review articles on basic and translational research into sleep and circadian rhythms. The journal focuses on topics covering the mechanisms of sleep/wake and circadian regulation from molecular to systems level, and on the functional consequences of sleep and circadian disruption. A key aim of the journal is the translation of basic research findings to understand and treat sleep and circadian disorders. Topics include, but are not limited to: Basic and translational research, Molecular mechanisms, Genetics and epigenetics, Inflammation and immunology, Memory and learning, Neurological and neurodegenerative diseases, Neuropsychopharmacology and neuroendocrinology, Behavioral sleep and circadian disorders, Shiftwork, Social jetlag.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


