电解质特性对离子通过固态纳米孔的影响:实验与模拟
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
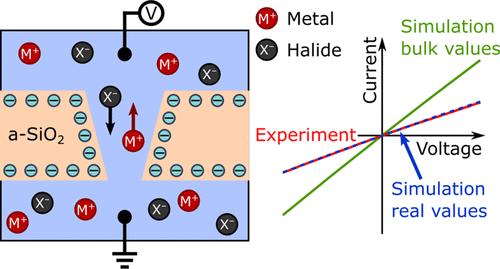
纳米孔膜技术用途广泛,可用于从清洁能源生产到过滤和传感等许多不同的应用领域。可以通过对系统进行数值模拟来提高性能,例如,研究纳米孔的几何形状或表面特性如何改变系统的离子传输行为或流体动力学。使用 COMSOL Multiphysics 等软件进行有限元分析(FEA)是一种广泛应用的数值模拟工具。我们发现,在有限元分析中实现电解质的普遍方法可能与物理精确值有很大偏差。通常假定盐分子完全解离,并忽略温度的影响。此外,离子的扩散系数以及流体的介电常数、密度和粘度值都被假定为无限稀释时的体积值。通过对具有锥形孔的无定形二氧化硅纳米孔膜进行电导实验,并用有限元分析法模拟孔隙系统,结果表明,对于浓度超过 100 mM 的不同一价和二价氯化物(LiCl、NaCl、KCl、MgCl2 和 CaCl2),普通假设并不成立。取而代之的是一种根据盐的类型和浓度实施所有参数的程序。对普通方法的这一修改提高了数值模拟的准确性,从而为纳米孔中的离子传输提供了更全面的见解,而这正是其他方法所缺乏的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Effect of Electrolyte Properties on Ionic Transport through Solid-State Nanopores: Experiment and Simulation
Nanopore membranes enable versatile technologies that are employed in many different applications, ranging from clean energy generation to filtration and sensing. Improving the performance can be achieved by conducting numerical simulations of the system, for example, by studying how the nanopore geometry or surface properties change the ionic transport behavior or fluid dynamics of the system. A widely employed tool for numerical simulations is finite element analysis (FEA) using software, such as COMSOL Multiphysics. We found that the prevalent method of implementing the electrolyte in the FEA can diverge significantly from physically accurate values. It is often assumed that salt molecules fully dissociate, and the effect of the temperature is neglected. Furthermore, values for the diffusion coefficients of the ions, as well as permittivity, density, and viscosity of the fluid, are assumed to be their bulk values at infinite dilution. By performing conductometry experiments with an amorphous SiO2 nanopore membrane with conical pores and simulating the pore system with FEA, it is shown that the common assumptions do not hold for different mono- and divalent chlorides (LiCl, NaCl, KCl, MgCl2, and CaCl2) at concentrations above 100 mM. Instead, a procedure is presented where all parameters are implemented based on the type of salt and concentration. This modification to the common approach improves the accuracy of the numerical simulations and thus provides a more comprehensive insight into ion transport in nanopores that is otherwise lacking.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


