苄基氧化膦/硫醚与 (COCl)2 的反应:合成新型酰基氯取代的氯膦酰化物
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
介绍了苄基氧化膦/硫化物与草酰氯的新反应。由此产生的活性中间体--酰基氯取代的氯膦酰化物,能够发生酯化和弗里德尔-卡夫酰化反应,最终生成 2-(2-溴苯基)-2-(二苯基磷酰)乙酸甲酯或 β-羰基-二元氧化膦衍生物。此外,当酰基氯取代的氯膦酰亚胺中含有炔基时,会发生分子内环化,从而形成一对反式和顺式二氯膦酰基苯并富烯异构体。我们利用 31P{1H} NMR 光谱仔细监测了酰基氯取代的氯膦酰亚胺的生成过程,并利用 31P{1H} NMR 光谱分析了氯膦酰亚胺的化学结构。核磁共振光谱仔细监测了酰基氯-取代的氯膦酰亚胺的生成过程,并提出了合理的反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
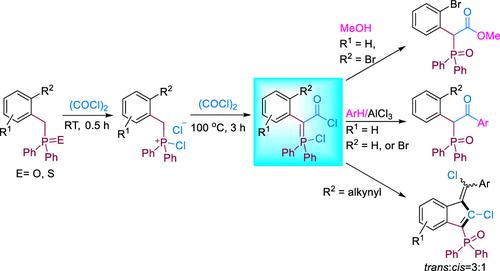
Reactions of Benzyl Phosphine Oxide/Sulfide with (COCl)2: Synthesis of Novel Acyl Chloride-Substituted Chlorophosphonium Ylides
New reactions of benzyl phosphine oxide/sulfide with oxalyl chloride are presented. The resulting reactive intermediates, acyl chloride-substituted chlorophosphonium ylides, are capable of undergoing esterification and Friedel–Crafts acylation reactions, ultimately yielding either methyl 2-(2-bromophenyl)-2-(diphenylphosphoryl)acetate or β-carbonyl-diarylphosphine oxide derivatives. Additionally, when an alkynyl group is contained in the acyl chloride-substituted chlorophosphonium ylide, intramolecular cyclization occurs, leading to the formation of a pair of trans- and cis-dichlorophosphonyl benzofulvene isomers. The generation process of acyl chloride-substituted chlorophosphonium ylide was carefully monitored by using 31P{1H} NMR spectroscopy, and a plausible reaction mechanism was proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


