利用三唑分子催化剂将二氧化碳电还原为甲烷
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
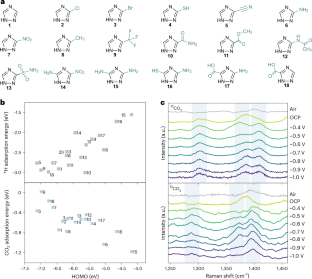
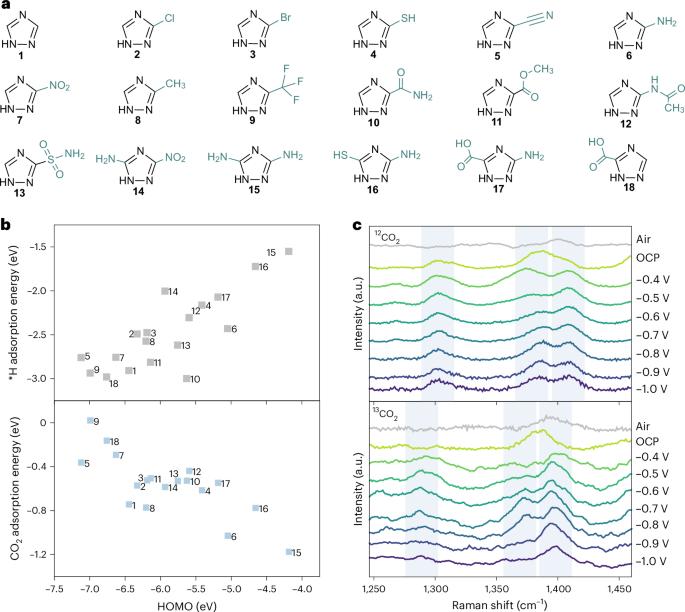
为获得增值燃料和原料而进行的电化学二氧化碳还原反应通常依赖于金属催化剂。与金属催化剂相比,有机分子催化剂具有更敏锐的可调性,但在长期运行的工业相关电流密度条件下,有机分子催化剂仍无法将二氧化碳催化成碳氢化合物,催化机理也仍然难以捉摸。在此,我们报告了基于 3,5-二氨基-1,2,4-三唑的膜电极组件在 250 mA cm-2 条件下将 CO2 转化为 CH4 的过程,其法拉第效率为 (52 ± 4)%,周转频率为 23,060 h-1。我们的机理研究表明,在 3,5-二氨基-1,2,4-三唑电极上,由于活性位点的空间分布和分子轨道的合适能级,二氧化碳通过中间产物 *CO2-*COOH-*C(OH)2-*COH 还原生成 CH4。在总电流为 10 A(电流密度 = 123 mA cm-2)的条件下运行 10 小时的试验系统能够以 23.0 mmol h-1 的速率产生 CH4。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electroreduction of CO2 to methane with triazole molecular catalysts
The electrochemical CO2 reduction reaction towards value-added fuel and feedstocks often relies on metal-based catalysts. Organic molecular catalysts, which are more acutely tunable than metal catalysts, are still unable to catalyse CO2 to hydrocarbons under industrially relevant current densities for long-term operation, and the catalytic mechanism is still elusive. Here we report 3,5-diamino-1,2,4-triazole-based membrane electrode assemblies for CO2-to-CH4 conversion with Faradaic efficiency of (52 ± 4)% and turnover frequency of 23,060 h−1 at 250 mA cm−2. Our mechanistic studies suggest that the CO2 reduction at the 3,5-diamino-1,2,4-triazole electrode proceeds through the intermediary *CO2–*COOH–*C(OH)2–*COH to produce CH4 due to the spatially distributed active sites and the suitable energy level of the molecular orbitals. A pilot system operated under a total current of 10 A (current density = 123 mA cm−2) for 10 h is able to produce CH4 at a rate of 23.0 mmol h−1. Electrochemical CO2 reduction to make fuels and feedstocks often relies on metal-based catalysts. Here the authors report membrane electrode assemblies operating with relatively high current densities for CO2-to-CH4 conversion using organic molecular catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


