从机理上深入了解含有动态希夫键的正交封端和无正交维聚苯并噁嗪的闭环可回收性
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
玻璃聚合物是可持续材料领域的一项重要突破,尤其是在塑料废物管理方面。这些聚合物可以设计成具有可逆链接,从而可以在不影响材料完整性的情况下进行键交换。因此,了解苯并恶嗪单体合成中使用的酚类前体与聚苯并恶嗪网络随后获得的三聚体特性之间的相关性,有助于我们最大限度地发挥这些可重复使用网络的潜力。本研究以正交封端苯酚(香兰素、4-羟基-3-甲氧基苯甲醛)和无正交封端苯酚(4-羟基苯甲醛)为醛前体,以对苯二胺为胺前体,促进了动态亚胺网络的形成。在形成亚胺网络之前,先利用酚基使用硬脂胺形成苯并噁嗪。含亚胺的苯并恶嗪单体随后会进一步加热以诱导聚合。在以香兰素为基础的聚苯并恶嗪(PVBI)中加入甲氧基会使交联密度降低到 414 kJ mol-1。相比之下,使用 4-羟基苯甲醛合成的聚苯并恶嗪(PHBI)的交联密度(4842 kJ mol-1)明显更高。在 25 °C 的温度下,PVBI 的储存模量为 115 ± 5 MPa,而 PHBI 的储存模量为 356 ± 5 MPa。流变特性表明,交联密度的降低会导致弛豫时间的大幅缩短。这是因为较低的交联密度允许较高的链流动性,从而促进了交换动力学。由于亚胺键之间的交换反应引起了结构的拓扑重组,PVBI 显示出显著的延展性,其应力松弛时间小于 4 秒,而 PHBI 在 120 °C 时的应力松弛时间为 200 秒。根据应力松弛图确定的活化能,PVBI 为 51 kJ mol-1,PHBI 为 68 kJ mol-1。在热压条件下进行的再加工研究表明,即使经过三个再成型周期,储能模量和 Tg 等机械性能仍保持一致。此外,在聚苯并恶嗪基体中加入亚胺键有助于实现闭环回收程序,即材料可以分解成更小的碎片,然后重新组装。回收研究表明,PVBI 维聚物样品保留了 85% 的贮存模量,这表明这些样品在闭环系统中具有很高的可回收性。经过再加工和化学回收的玻璃聚合物材料在热性能和机械性能方面都有显著的保留。本文章由计算机程序翻译,如有差异,请以英文原文为准。
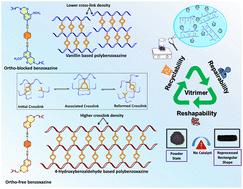
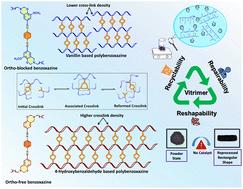
Mechanistic insights into ortho-blocked and ortho-free vitrimeric polybenzoxazines incorporating dynamic Schiff linkages for closed-loop recyclability†
Vitrimers are an important breakthrough in the field of sustainable materials, particularly within the context of plastic waste management. These polymers can be designed with reversible linkages, which allows them to undergo bond exchange processes without compromising the integrity of the material. Hence, comprehending the correlation between the phenolic precursor used in benzoxazine monomer synthesis and subsequent vitrimeric properties obtained in polybenzoxazine networks can aid us in maximizing the potential of these reusable networks. This work presents an investigation using an ortho-blocked phenol (vanillin, 4-hydroxy-3-methoxybenzaldehyde) and an ortho-free phenol (4-hydroxybenzaldehyde) as aldehyde precursors, together with p-phenylenediamine as an amine precursor, facilitating the formation of dynamic imine networks. Before the formation of the imine network, phenolic groups were utilized for the formation of benzoxazines using stearylamine. The imine-containing benzoxazine monomers were subsequently subjected to further heating to induce polymerization. The inclusion of a methoxy group in a vanillin-based polybenzoxazine (PVBI) results in a reduced crosslinking density of 414 kJ mol−1. In contrast, the polybenzoxazine (PHBI) synthesized using 4-hydroxybenzaldehyde exhibited a significantly higher crosslinking density (4842 kJ mol−1). At a temperature of 25 °C, the storage modulus of PVBI was 115 ± 5 MPa, while the storage modulus of PHBI was 356 ± 5 MPa. The rheological properties demonstrate that a decrease in crosslinking density leads to a substantial reduction in relaxation time. This is because a lower crosslinking density allows higher chain mobility, facilitating exchange kinetics. Due to the topological reconfiguration of the structure induced by the exchange reaction between imine bonds, PVBI demonstrated remarkable malleability, having a stress relaxation time of less than 4 seconds, while PHBI had a stress relaxation time of 200 seconds at 120 °C. The activation energy determined from stress relaxation plots was 51 kJ mol−1 for PVBI and 68 kJ mol−1 for PHBI. The reprocessing studies conducted under hot-pressing showed that the mechanical properties, such as storage modulus and Tg, remained consistent even after three remolding cycles. Furthermore, incorporating imine bonds in the polybenzoxazine matrix facilitated a closed-loop recycling procedure where the material can be broken down into smaller fragments and then reassembled. After conducting recycling investigations, it was shown that 85% of the storage modulus was preserved in PVBI vitrimeric samples, indicating that these samples exhibit a significant degree of recyclability within a closed-loop system. The reprocessed and chemically recycled vitrimeric materials demonstrate significant preservation of thermal and mechanical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


