小鼠大脑单细胞 m6A 图谱揭示细胞类型特异性 RNA 甲基组和年龄依赖性差异甲基化
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
N6-甲基腺苷(m6A)是大脑中一种丰富的mRNA修饰,在神经发育和大脑功能中具有重要作用。然而,由于技术限制,目前还无法对构成大脑的各个细胞类型中的 m6A 位点进行全面分析。在这里,我们开发了一种小鼠模型,可以在任何感兴趣的组织中以单细胞分辨率进行全转录组 m6A 检测。我们利用这些小鼠绘制了不同脑区和小鼠皮层单细胞内的 m6A 图谱,并发现了不同脑区和细胞类型之间高度共享的甲基化。不过,我们也在神经元中发现了少量不同甲基化的 mRNA,这些 mRNA 编码神经元信号传导的重要调控因子,我们还发现小胶质细胞的 m6A 水平低于其他细胞类型。最后,我们在老龄小鼠体内进行了单细胞 m6A 图谱绘制,发现了许多 m6A 变化随年龄变化的转录本。本文章由计算机程序翻译,如有差异,请以英文原文为准。
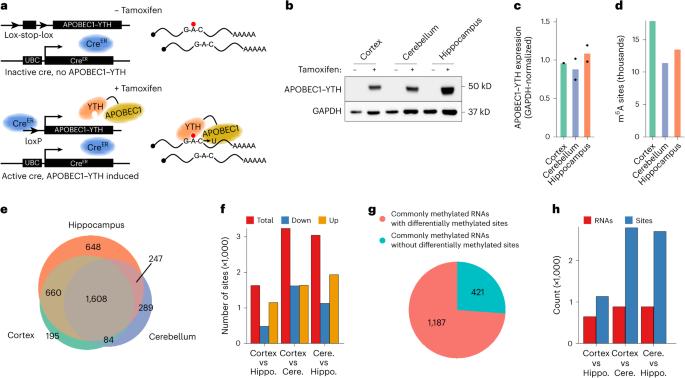
Single-cell m6A profiling in the mouse brain uncovers cell type-specific RNA methylomes and age-dependent differential methylation
N6-methyladenosine (m6A) is an abundant mRNA modification in the brain that has important roles in neurodevelopment and brain function. However, because of technical limitations, global profiling of m6A sites within the individual cell types that make up the brain has not been possible. Here, we develop a mouse model that enables transcriptome-wide m6A detection in any tissue of interest at single-cell resolution. We use these mice to map m6A across different brain regions and within single cells of the mouse cortex and discover a high degree of shared methylation across brain regions and cell types. However, we also identify a small number of differentially methylated mRNAs in neurons that encode important regulators of neuronal signaling, and we discover that microglia have lower levels of m6A than other cell types. Finally, we perform single-cell m6A mapping in aged mice and identify many transcripts with age-dependent changes in m6A. The authors perform the first single-cell profiling of m6A in the mouse brain. They uncover relative hypomethylation of microglial mRNA compared to other cell types, and they identify hundreds of RNAs that undergo differential methylation with age.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


