表转录组的动态变化揭示了水稻在病原体感染期间由ac4C引导的翻译重编程过程
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
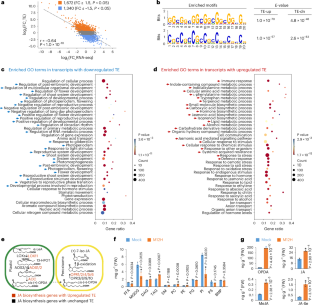
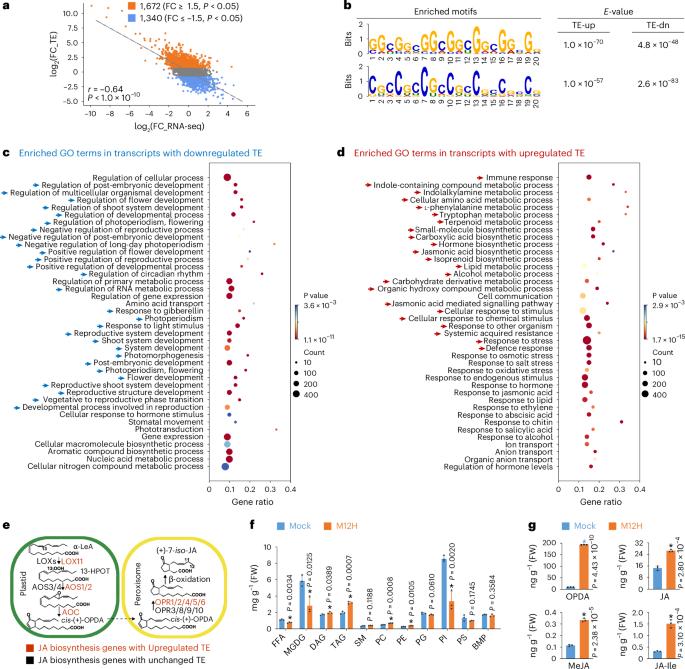
信使 RNA 修饰在 RNA 生物学中起着举足轻重的作用,但在植物免疫反应中,表转录组的综合景观变化在很大程度上仍不为人所知。在这里,我们报告了病原体挑战时由 ac4C mRNA 修饰引导的翻译重编程。我们首先研究了翻译组和表转录组的动态变化,发现单碱基分辨率的 ac4C 变化促进了木格氏球菌(Magnaporthe oryzae)感染后的翻译重编程。然后,通过表征 m1A、2'O-Nm、ac4C、m5C、m6A 和 m7G 的特定分布,我们发现 ac4C 与其他修饰不同,富集在密码子的第 3 位,从而稳定了 Watson-Crick 碱基配对。重要的是,我们证明了在病原体感染时,ac4C 作家 OsNAT10/OsACYR(RNA 中的 N-ACETYLTRANSFERASE FOR CYTIDINE)表达的增加会促进翻译,从而促进免疫反应的快速激活,包括茉莉酸生物合成的增强。我们的研究提供了一个 mRNA 修饰图谱,有助于深入了解 ac4C 在植物免疫中的功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamics of epitranscriptomes uncover translational reprogramming directed by ac4C in rice during pathogen infection
Messenger RNA modifications play pivotal roles in RNA biology, but comprehensive landscape changes of epitranscriptomes remain largely unknown in plant immune response. Here we report translational reprogramming directed by ac4C mRNA modification upon pathogen challenge. We first investigate the dynamics of translatomes and epitranscriptomes and uncover that the change in ac4C at single-base resolution promotes translational reprogramming upon Magnaporthe oryzae infection. Then by characterizing the specific distributions of m1A, 2’O-Nm, ac4C, m5C, m6A and m7G, we find that ac4Cs, unlike other modifications, are enriched at the 3rd position of codons, which stabilizes the Watson–Crick base pairing. Importantly, we demonstrate that upon pathogen infection, the increased expression of the ac4C writer OsNAT10/OsACYR (N-ACETYLTRANSFERASE FOR CYTIDINE IN RNA) promotes translation to facilitate rapid activation of immune responses, including the enhancement of jasmonic acid biosynthesis. Our study provides an atlas of mRNA modifications and insights into ac4C function in plant immunity. By characterizing the dynamics of epitranscriptomes and translatomes upon Magnaporthe oryzae infection, the authors uncover that ac4C enriched in the 3rd nt of codons improves translation of mRNA to enhance rice resistance against M. oryzae.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


