在 Co3O4 上进行原位面转化工程,实现硝酸盐到氨的高效电还原
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
不同的暴露面会导致催化活性的巨大差异。在此,我们制备了具有{112}、{112}&{111}和{111}暴露面的 Co3O4 六方纳米片,用于电化学硝酸盐还原反应(NO3RR)。通过原位电化学表征和理论分析,阐明了硝酸还原反应在 Co3O4 {111} 和 {112} 面上的反应途径。当 Co3O4 的主导面从 {112} 转变为 {111} 时,速率决定步骤从 *NO2 → *NO2H 转变为 *NO3H → *NO2,能垒降低到 0.48 eV。{111}面促进了 NOx 和 NHx 中间产物的氢化。值得注意的是,Co3O4-{111}催化剂具有优异的 NO3RR 性能,NH3 产率达到 5.73 mg mgcat.-1 h-1,超过了大多数已报道的活性催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
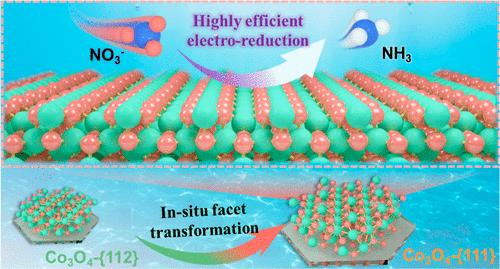
In Situ Facet Transformation Engineering over Co3O4 for Highly Efficient Electroreduction of Nitrate to Ammonia
Various exposed facets can cause a huge difference in the catalytic activity. Here we prepared Co3O4 hexagonal nanosheets with exposed {112}, {112}&{111}, and {111} facets for the electrochemical nitrate reduction reactions (NO3RR). The reaction pathways of the NO3RR on Co3O4 {111} and {112} facets are clarified through in situ electrochemical characterizations and theoretical analysis. As the dominating facet of Co3O4 transforms from {112} to {111}, the rate-determining step changes from *NO2 → *NO2H to *NO3H → *NO2, with the energy barrier decreasing to 0.48 eV. And the {111} facet promotes the hydrogenation of NOx and NHx intermediates. Notably, the Co3O4-{111} catalyst shows exceptional NO3RR performance, achieving an NH3 yield of 5.73 mg mgcat.–1 h–1, surpassing the majority of the reported activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


