狨猴和人类滋养层干细胞的信号需求不同,再现了滋养层侵入的不同模式
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
由于无法获得早期胚胎,人类滋养细胞的早期发育一直难以捉摸。非人灵长类动物模型再现了人类发育的许多特征,并能进入着床后的早期阶段。在这里,我们追踪了体内浅表植入狨猴胚胎滋养层细胞系从植入前到植入后的转变。我们将狨猴天真多能干细胞分化成滋养层干细胞(TSCs),它们表现出滋养层特有的转录组、甲基组、分化潜能和长期自我更新能力。值得注意的是,人类滋养层干细胞的培养条件并不支持狨猴滋养层干细胞的衍生,反而会诱导狨猴细胞的胚外中胚层样命运。我们的研究表明,联合抑制MEK、TGF-β/NODAL和组蛋白去乙酰化酶可稳定狨猴TSC的着床前滋养母细胞样特征。与此相反,这些条件使人类 TSCs 向滋养细胞外分化。我们的研究为利用植入策略的进化差异来阐明人类滋养细胞的发育和入侵提供了一个范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
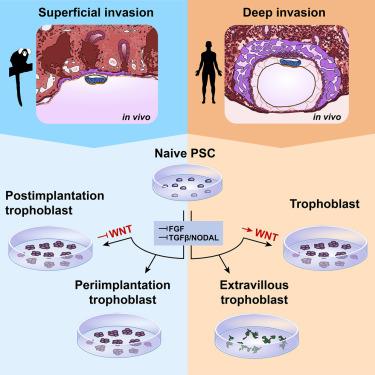
Marmoset and human trophoblast stem cells differ in signaling requirements and recapitulate divergent modes of trophoblast invasion
Early human trophoblast development has remained elusive due to the inaccessibility of the early conceptus. Non-human primate models recapitulate many features of human development and allow access to early postimplantation stages. Here, we tracked the pre- to postimplantation transition of the trophoblast lineage in superficially implanting marmoset embryos in vivo. We differentiated marmoset naive pluripotent stem cells into trophoblast stem cells (TSCs), which exhibited trophoblast-specific transcriptome, methylome, differentiation potential, and long-term self-renewal. Notably, human TSC culture conditions failed to support marmoset TSC derivation, instead inducing an extraembryonic mesoderm-like fate in marmoset cells. We show that combined MEK, TGF-β/NODAL, and histone deacetylase inhibition stabilizes a periimplantation trophoblast-like identity in marmoset TSCs. By contrast, these conditions differentiated human TSCs toward extravillous trophoblasts. Our work presents a paradigm to harness the evolutionary divergence in implantation strategies to elucidate human trophoblast development and invasion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


