异喹啉鎓盐的非对映选择性脱芳香双官能化,获得桥联四氢异喹啉†。
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们报告了一种非对映选择性脱芳香双官能化策略,利用异喹啉盐获得桥联四氢异喹啉。该策略依靠战略性地使用烯和胺作为反应伙伴,在原位生成双亲核物,以便随后进行脱芳烃反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
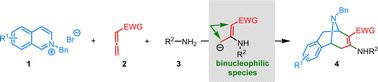
Diastereoselective dearomative bifunctionalization of isoquinolinium salts to access bridged tetrahydroisoquinolines†
Herein, we report a diastereoselective dearomative bifunctionalization strategy of using isoquinolinium salts to access bridged tetrahydroisoquinolines. The strategy relies on the strategic use of allenes and amines as reaction partners to generate binucleophilic species in situ for subsequent dearomatization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


