紫外线和可见光照射下富氧氯化铋 Bi24O31Cl10 光催化剂对罗丹明 B 染料的光催化降解:途径和机制
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
光催化去除废水中的有机污染物因其对环境和生态的重要意义而备受关注。本研究采用单步固态反应合成了富铋氧氯化铋(Bi24O31Cl10),并将其作为光催化剂用于降解水溶液中的罗丹明 B。该光催化剂是通过环保固态法制备的,将氧化铋(Bi2O3)和氧氯化铋(BiOCl)混合,然后在 600 °C 下直接退火。合成材料通过多种技术进行了表征,包括 X 射线衍射 (XRD)、傅立叶变换红外 (FTIR)、拉曼光谱、扫描电子显微镜 (SEM)、布鲁诺-艾美特-泰勒 (BET) 表面积分析、能量色散 X 射线光谱 (EDS)、漫反射、紫外-可见光和光致发光 (PL) 光谱。里特维尔德精炼证实形成了空间群为 P2/c 的纯单斜 Bi24O31Cl10 相,XRD 图谱显示材料结晶良好。扫描电子显微镜(SEM)显示了微米大小的结晶,而 BET 表面积分析显示其值为 22.546 m²/g,表明较大的表面积可提高光催化性能。Bi24O31Cl10 的光催化活性通过罗丹明 B(RhB)的降解得到了证明,在紫外光下,90 分钟内即可实现完全降解。在可见光下,180 分钟后降解效率达到 98%。动力学研究表明,降解遵循伪一阶模型。在溶液 pH 值为 5、催化剂浓度为 1 克/升、染料浓度为 5 毫克/升的条件下,降解效果达到最佳。值得注意的是,该光催化剂具有极佳的重复利用率,在五个循环中都能保持较高的效率,仅从 100% 到 90% 稍有下降。该研究提出了可能的反应机理,并利用液相色谱-质谱法(LC-MS)对降解产物进行了监测,从而阐明了 RhB 的降解途径。此外,还测试了光催化剂对甲基橙(MO)和亚甲蓝(MB)的有效性,在紫外线照射下,MO 和 MB 的降解率分别达到近 100%和 41%。这项研究凸显了 Bi24O31Cl10 作为光催化剂在废水处理中降解染料的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
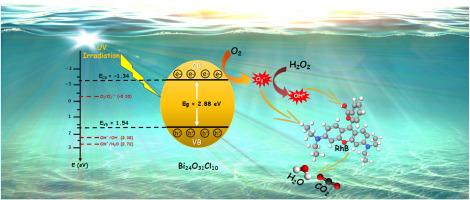
Photocatalytic degradation of Rhodamine B dye over oxygen-rich bismuth oxychloride Bi24O31Cl10 photocatalyst under UV and Visible light irradiation: Pathways and mechanism
Photocatalytic removal of organic pollutants from wastewater has recently garnered significant attention due to its environmental and ecological significance. In this study, a bismuth-rich bismuth oxychloride (Bi24O31Cl10) was synthesized using a single-step solid-state reaction and applied as a photocatalyst for the degradation of rhodamine B in aqueous solution. The photocatalyst was prepared through an eco-friendly solid-state method by mixing bismuth oxide (Bi2O3) and bismuth oxychloride (BiOCl), followed by direct annealing at 600 °C. The synthesized material was characterized through various techniques, including X-ray diffraction (XRD), Fourier-transform infrared (FTIR), Raman spectroscopy, scanning electron microscopy (SEM), Brunauer-Emmett-Teller (BET) surface area analysis, energy dispersive X-ray spectroscopy (EDS), diffuse reflectance, UV–Visible, and photoluminescence (PL) spectroscopies. The electronic structure of the Bi24O31Cl10 bulk material was analyzed using Density Functional Theory (DFT).
Rietveld refinement confirmed the formation of a pure monoclinic Bi24O31Cl10 phase with the space group P2/c, and the XRD patterns indicated well-crystallized material. SEM revealed micron-sized crystallites, while BET surface area analysis showed a value of 22.546 m²/g, suggesting that a larger surface area could enhance photocatalytic performance. The band gap of the material was determined to be 2.88 eV, with an absorption edge at 430 nm, indicating a promising response to visible light.
The photocatalytic activity of Bi24O31Cl10 was demonstrated by the degradation of rhodamine B (RhB), with complete degradation achieved in 90 min under UV light. Under visible light, 98 % degradation efficiency was reached after 180 min. Kinetic studies showed that the degradation followed a pseudo-first-order model. Optimal conditions for maximum degradation were found at a solution pH of 5, a catalyst concentration of 1 g/L, and a dye concentration of 5 mg/L. Remarkably, the photocatalyst exhibited excellent reusability, maintaining high efficiency over five cycles, with only a slight decrease from 100 % to 90 %.
Trapping experiments identified that reactive species such as superoxide radicals (∙O2−) and hydroxyl radicals (∙OH) played key roles in the photocatalytic process. The possible reaction mechanism was proposed, and degradation products were monitored using liquid chromatography-mass spectrometry (LC-MS), allowing for the elucidation of the degradation pathways of RhB. Additionally, the photocatalyst's effectiveness was tested on Methyl Orange (MO) and Methylene Blue (MB), achieving nearly 100 % degradation for MO and 41 % for MB under UV light. This research highlights the significant potential of Bi24O31Cl10 as a photocatalyst for dye degradation in wastewater treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


