枸杞类胡萝卜素裂解二氧酶 1 (LbCCD1) 的分子克隆、表达和生化鉴定
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
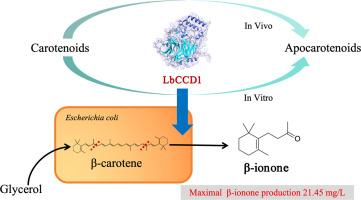
类胡萝卜素裂解二氧酶(CCDs)在挥发性类胡萝卜素的生物合成过程中发挥着关键作用,由于其芳香和生物活性特性,类胡萝卜素具有重要的工业应用价值。本研究的重点是枸杞中 LbCCD1 基因的分子克隆和生化鉴定。LbCCD1 基因被成功克隆并在大肠杆菌中异源表达,并以几种类胡萝卜素为底物,通过体内外实验研究其特异性。LbCCD1 蛋白能够在体外裂解多种类胡萝卜素,包括 β-胡萝卜素、玉米黄质、虾青素和 β-apo-8′-carotenal 的 9、10(9′,10′)双键,分别生成 β-酮、3-羟基-4-氧代-β-酮和 3-羟基-β-酮。在积累类胡萝卜素的大肠杆菌中,LbCCD1 还能在 9、10(9′、10′)双键处裂解玉米黄素和 β-胡萝卜素,分别生成 β-酮。有趣的是,与其他 CCD1 家族的酶不同,LbCCD1 在体内和体外都没有表现出对番茄红素的裂解活性。在之前的实验中,我们证实 LbCCD1 在体外对 β-apo-8′- 胡萝卜烯醛具有裂解活性,因此我们使用 β-apo-8′- 胡萝卜烯醛作为底物来表征酶的特性。在这样的条件下(温度 24 ℃,IPTG 0.1 mM,诱导时间 24 h),LbCCD1 的表达得到了优化。LbCCD1 的生化特性分析表明,在 pH 值为 9 和温度为 55 ℃ 时,LbCCD1 的活性最佳。加入 10 % 的乙醇可使酶活性提高到 15 % 以上。然而,Fe2+ 的浓度对酶活性的影响很小。β-apo-8′-胡萝卜烯醛的 Vmax 为 8.6 U/mg ,Km 为 0.27 mM。为了初步验证 LbCCD1 作为生产 β-酮的生物成分的潜力。通过将 LbCCD1 引入高产β-胡萝卜素的底盘细胞并优化条件(温度 30 °C、IPTG 0.01 mM、Fe2+ 浓度 0.05 mM),β-酮产量达到 21.45 mg/L。这项研究的重点是木本植物衍生的 CCDs 之一,该领域的研究相对较少。它为扩大 CCD 酶库,确定合适的 CCD 以及研究 CCD 的结构-功能关系奠定了基础。此外,它还为新型 CCD 基因工程和开发 CCD 作为生物催化剂和合成生物学平台的高级应用奠定了基础。这些进展将有助于从类胡萝卜素中高效生产挥发性芳香化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular cloning, expression, and biochemical characterization of carotenoid cleavage dioxygenase 1 (LbCCD1) from Lycium barbarum
Carotenoid cleavage dioxygenases (CCDs) play a pivotal role in the biosynthesis of volatile apocarotenoids, which have significant industrial applications due to their aromatic and bioactive properties. This study focuses on the molecular cloning and biochemical characterization of the LbCCD1 from Lycium barbarum. The LbCCD1 gene was successfully cloned and heterologously expressed in Escherichia coli and several carotenoids were using as substrates for investigate its specificity by in vitro and in vivo experiment. The LbCCD1 protein was able to cleave a variety of carotenoids including β-carotene, zeaxanthin, astaxanthin, and β-apo-8′-carotenal, at the 9, 10 (9′, 10′) double bond to produce β-ionone, 3‑hydroxy-4-oxo-β-ionone, and 3‑hydroxy-β-ionone, respectively in vitro. LbCCD1 could also cleave zeaxanthin and β-carotene at the 9, 10 (9′, 10′) double bond to produce β-ionone, respectively, in E. coli accumulating carotenoids. Interestingly, LbCCD1 did not exhibit cleavage activity on lycopene either in vivo or in vitro unlike other CCD1 family enzymes.
In the previous experiment, it was confirmed that LbCCD1 exhibits cleavage activity towards β-apo-8′-carotenal in vitro, so we used β-apo-8′-carotenal as the substrate for characterizing the enzymatic properties. The expression of LbCCD1 was optimized at such conditions (temperature 24 °C, IPTG 0.1 mM, induction time 24 h). The biochemical characterization of LbCCD1 revealed the optimal activities were at pH 9 and 55 °C. The addition of 10 % ethanol could increase enzyme activity to above 15 %. However, the concentration of Fe2+ has a minimal effect on enzyme activity. The Vmax for β-apo-8′-carotenal was 8.6 U/mg, while the Km was 0.27 mM. To preliminarily verify the potential of LbCCD1 as a biological component for β-ionone production. By introducing LbCCD1 into the β-carotene-high-producing chassis cell and optimizing the conditions (temperature 30 °C, IPTG 0.01 mM, Fe2+ concentration 0.05 mM), the β-ionone yield reached 21.45 mg/L. This study focused on one of the CCDs derived from woody plants, which have been relatively underexplored. It lays the groundwork for expanding the CCD enzyme library, identifying suitable CCDs, and investigating the structure-function relationship of CCDs. Furthermore, it sets the stage for engineering novel CCD genes and developing advanced applications of CCDs as biocatalysts and platforms for synthetic biology. These advancements will enable the efficient production of volatile aroma compounds from carotenoids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


