蔗糖棕榈酰酯化反应的强化和选择性控制
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
蔗糖长链脂肪酸酯是一种非离子表面活性剂,在食品、化妆品和制药领域有着重要的应用。用化学和生物催化方法合成这些酯类涉及到协调反应效率和选择性的难题。在这里,我们展示了在没有或有固定化脂肪酶(南极念珠菌;兰努吉诺斯热酵母菌)的情况下,在含有不同量的 1-丁基-3-甲基咪唑醋酸酯([Bmim][OAc])离子液体(体积比为 5-60%)的干燥 2-甲基-2-丁醇(2M2B)中,棕榈酸乙烯酯(≥ 1 摩尔当量)对蔗糖(200 mM;∼70 g/L)的酯交换反应。由于[Bmim][OAc]对蔗糖溶解度和催化速率加速的综合作用,[Bmim][OAc](≥ 20 %(体积))足以有效促进酯交换反应,而酶没有提供额外的益处,甚至对产物选择性和初始速率也没有影响。使用≥ 2 摩尔当量的棕榈酸乙烯酯,在 60°C 下 72 小时内,棕榈酰供体的水解程度较低(∼7%),蔗糖几乎完全转化(93%),得到的蔗糖酯产物中含有∼75%的单酯。其他用于碳水化合物(反式)酯化反应强化的一般策略,如在有机溶剂(此处:2M2B 和 20 Vol.% DMSO)中对底物进行微分散或使用低溶剂条件,在蔗糖方面的效率较低,转化率和单酯选择性均不高。总之,这项研究表明,[Bmim][OAc]驱动合成棕榈酰蔗糖(单)酯的反应强化(产物≥ 110 克/升;生产率≥ 1.5 克/升小时)达到了理想的亲水-亲油平衡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
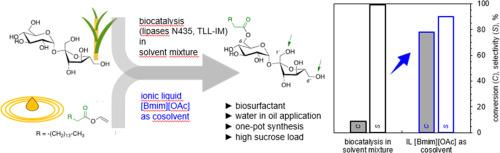
Reaction intensification and selectivity control for palmitoyl transesterification of sucrose
Long-chain fatty acid esters of sucrose are nonionic surfactants with important applications in food, cosmetics and pharmacy. Their synthesis by chemical and biocatalytic methods involves the difficult task of coordinating efficiency and selectivity of the reaction used. Here, we show transesterification of sucrose (200 mM; ∼70 g/L) from vinyl palmitate (≥ 1 mole equivalent) in dry 2-methyl-2-butanol (2M2B) containing variable amount of 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) ionic liquid (5–60 % by volume), in the absence or presence of immobilized lipase (Candida antarctica; Thermomyces lanuginosus). Due to its combined effect on sucrose solubility and catalytic rate acceleration, the [Bmim][OAc] (≥ 20 % by volume) was sufficient to promote the transesterification efficiently, with no additional benefit provided by the enzyme, not even on the product selectivity and the initial rate. Using ≥ 2 mole equivalents of vinyl palmitate, sucrose was converted nearly fully (93 %) at low hydrolysis of the palmitoyl donor (∼7 %) in 72 h at 60°C, giving sucrose ester product comprised of ∼75 % monoester. Other general strategies of reaction intensification for carbohydrate (trans)esterification, such as substrate microdispersion in organic solvent (here: 2M2B with 20 vol.% DMSO) or usage of low-solvent conditions, proved by far less efficient with sucrose, failing in conversion and monoester selectivity. Overall, this study shows reaction intensification (product ≥ 110 g/L; productivity ≥ 1.5 g/L h) for [Bmim][OAc]-driven synthesis of palmitoyl sucrose (mono)esters of desired hydrophilic-lipophilic balance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


