作为无机核酸酶的硫代氨基羰基二氧钼 (VI) 复合物
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究侧重于从 5-溴水杨醛和取代的硫代氨基甲酸烷基酯中设计出烷基取代的硫代氨基甲酸烷基酯以及相应的二噁钼(VI)配合物。除了元素分析之外,还通过物理化学和光谱技术对合成的配体和相应的配合物进行了进一步分析。DNA 相互作用研究基于紫外吸收滴定法和凝胶电泳法。发现配体(L1-L4)的结合常数(Kb)分别为 1.20 ± 0.1 × 104 M-1;1.32 ± 0.2 × 104 M-1;1.27 ± 0.3 × 104 M-1 和 1.25 ± 0.复合物(C1-C4)的结合力分别为 1.45 ± 0.2 × 104 M-1;2.10 ± 0.4 × 104 M-1;1.54 ± 0.2 × 104 M-1 和 2.13 ± 0.2 × 104 M-1。与配体相比,复合物显示出更高的结合倾向。此外,凝胶电泳研究结果表明,配体显示出缺口环状(形式 I)的裂解模式。然而,复合物的裂解模式既有切口环状的,也有超卷曲的,分别为形式 I & II。这意味着配体和复合物与 DNA 的结合效力不同,因此裂解模式也不同。本文章由计算机程序翻译,如有差异,请以英文原文为准。
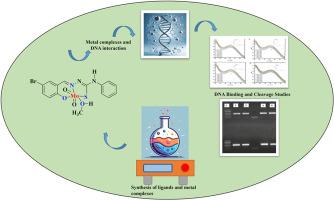
Thiosemicarbazone-based Dioxomolybdenum (VI) complexes as inorganic nucleases
This study focuses on the design of alkyl-substituted thiosemicarbazones from 5-bromosalicylaldehyde and substituted thiosemicarbazides and the corresponding dioxomolybdenum(VI) complexes, hitherto unreported. In addition to elemental analyses, the synthesized ligands and the corresponding complexes are further analyzed by physico-chemical and spectroscopic techniques. The DNA interaction studies are based on UV absorption titration and gel electrophoretic methods. The binding constants (Kb) for the ligands (L1-L4) are found to be 1.20 ± 0.1 × 104 M−1; 1.32 ± 0.2 × 104 M−1; 1.27 ± 0.3 × 104 M−1 and 1.25 ± 0.3 × 104 M−1 respectively and that of the complexes (C1–C4) are 1.45 ± 0.2 × 104 M−1; 2.10 ± 0.4 × 104 M−1; 1.54 ± 0.2 × 104 M−1 and 2.13 ± 0.2 × 104 M−1, respectively. The complexes have shown relatively higher binding propensity as compared to that of the ligands. Further, as the outcome of gel electrophoresis studies, the ligands show a cleavage pattern of nicked circular (Form I). However, the complexes show cleavage patterns of both nicked circular and supercoiled, Forms I & II respectively. This implies that both the ligands and complexes possess varying binding efficacy with DNA and accordingly the cleavage patterns also differ.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


