液滴蒸发过程中的对流对 CFRP-AA2024 电偶中腐蚀产物分布的影响:建模和实验方法
IF 7.4
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
这项研究旨在阐明电解质液滴在蒸发过程中产生的对流对 CFRP-AA2024 系统中 Al(OH)3 分布的影响。我们采用了有限元方法和实验方法来确定对流是显著还是可忽略不计。通过将 Navier-Stokes 和 Nernst-Planck 方程与适当的几何和边界条件相耦合,对腐蚀产物的瞬态分布进行了数值跟踪。结果表明,Al(OH)3 会优先沉积在 CFRP 表面,而在排除对流的情况下则不会出现这种情况。实验证实,内部对流可以传输 Al(OH)3 复合物,这与数值预测结果一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
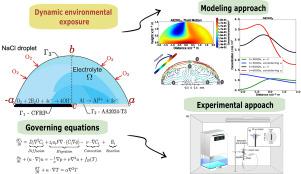
Influence of convection during droplet evaporation on corrosion product distribution in CFRP-AA2024 galvanic couple: Modeling and experimental approaches
This work aims to clarify the impact of convection, generated within an electrolyte droplet during evaporation, on the distribution of in the CFRP-AA2024 system. Both Finite Element and experimental approaches were employed to determine whether convection is significant or negligible. By coupling the Navier–Stokes and Nernst–Planck equations with appropriate geometric and boundary conditions, the transient distribution of corrosion products was numerically tracked. The results showed that preferentially deposits on the CFRP surface, a pattern absent when convection is excluded. Experiments confirmed that internal convective currents can transport the compound, aligning with the numerical predictions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Corrosion Science
工程技术-材料科学:综合
CiteScore
13.60
自引率
18.10%
发文量
763
审稿时长
46 days
期刊介绍:
Corrosion occurrence and its practical control encompass a vast array of scientific knowledge. Corrosion Science endeavors to serve as the conduit for the exchange of ideas, developments, and research across all facets of this field, encompassing both metallic and non-metallic corrosion. The scope of this international journal is broad and inclusive. Published papers span from highly theoretical inquiries to essentially practical applications, covering diverse areas such as high-temperature oxidation, passivity, anodic oxidation, biochemical corrosion, stress corrosion cracking, and corrosion control mechanisms and methodologies.
This journal publishes original papers and critical reviews across the spectrum of pure and applied corrosion, material degradation, and surface science and engineering. It serves as a crucial link connecting metallurgists, materials scientists, and researchers investigating corrosion and degradation phenomena. Join us in advancing knowledge and understanding in the vital field of corrosion science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


