基于聚合物纳米凝胶的治疗性纳米疫苗,可通过全周期免疫调节预防和直接治疗肿瘤
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
构建一种既能激活免疫细胞又能有效治疗肿瘤的癌症纳米疫苗仍是一项挑战。在此,我们展示了一种基于聚(N-乙烯基己内酰胺)纳米凝胶(NGs)的先进治疗性纳米疫苗配方的概念验证。凝胶上还涂有凋亡癌细胞膜(AM)。一方面,由于免疫原性增强,AM 可促进淋巴结中的抗原呈递细胞(APCs)识别 NG,然后负载的 Mn 和 cGAMP 可通过激活干扰素基因刺激因子(STING)使 APCs 成熟,从而触发免疫以阻止肿瘤生长。另一方面,在超声波照射肿瘤时,NGs 可选择性地释放 Mn2+ 产生羟基自由基,释放 Ce6 产生单氧,从而发挥局部化学动力学/声动力学疗法的作用,诱导免疫原性细胞死亡(ICD)。此外,Mn2+还能激活肿瘤中的STING,与ICD协同作用,增强免疫反应。总之,基于仿生 NG 的治疗性纳米疫苗既能直接唤起免疫系统,又能对肿瘤进行局部治疗,进一步激活 ICD,从而实现全周期免疫调节(ICD/抗原产生的肿瘤杀伤,以及肿瘤细胞/APCs 的免疫激活),以应对肿瘤的双侧生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
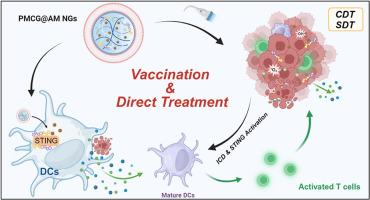
A polymer nanogel-based therapeutic nanovaccine for prophylaxis and direct treatment of tumors via a full-cycle immunomodulation
Construction of a cancer nanovaccine that can simultaneously activate immune cells and exert efficient tumor treatment still remains a challenge. Herein, we showcase a proof-of-concept demonstration of an advanced therapeutic nanovaccine formulation based on poly(N-vinylcaprolactam) nanogels (NGs) which were loaded with manganese dioxide (MnO2), the sonosensitizer chlorin e6 (Ce6), and the immune adjuvant cyclic GMP-AMP (cGAMP). The gels were furthermore coated with apoptotic cancer cell membranes (AM). On the one hand, the AM promoted the recognition of NGs by antigen presenting cells (APCs) in lymph nodes due to their enhanced immunogenicity, then the loaded Mn and cGAMP could mature APCs via stimulator of interferon genes (STING) activation for triggering immunity to prevent tumor growth. On the other hand, the NGs could selectively release Mn2+ for hydroxyl radical production and Ce6 to generate single oxygen under ultrasound irradiation of tumors, respectively, thereby exerting local chemodynamic/sonodynamic therapy to induce immunogenic cell death (ICD). Moreover, the Mn2+ could also activate STING in tumors to synergize with ICD for potentiated immune responses. Overall, the biomimetic NG-based therapeutic nanovaccine could directly evoke immune system, and also conduct local tumor treatment to further activate ICD, thus realizing a full-cycle immunomodulation (tumor killing for ICD/antigen production, and tumor cells/APCs immune activation) to tackle bilateral tumor growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


