超声产生的气泡能增强复合胶原水凝胶中间充质基质细胞的成骨分化能力
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
水凝胶可提供模拟细胞外基质的关键生物物理线索,从而改善间充质基质细胞(MSCs)的输送。间充质干细胞的分化依赖于刚度和粘弹性等生物物理线索,但传统的水凝胶在制造和植入后无法动态改变,无法积极引导分化。我们开发了一种由 I 型胶原蛋白和相移乳液组成的复合水凝胶,可利用超声波无创调节间充质干细胞的成骨分化。当暴露于超声波时,水凝胶中的乳液会非热气化成气泡,从而局部压实和硬化每个气泡周围的胶原基质。气泡的生长与基质的压实相关,气泡近端(即≤ ∼60 μm)的胶原区域的杨氏模量比远端(即 > ∼60 μm)增加了 2.5 倍。包裹在复合水凝胶中的间充质干细胞的活力和增殖不受气泡形成的影响。体外和体内研究显示,与远端区域相比,气泡近端胶原区域包裹的间充质干细胞显示出明显升高的成骨分化标志物 RUNX2 和骨钙素水平。此外,气泡附近的碱性磷酸酶活性和钙沉积也有所增强。间充质干细胞干性标志物CD90则呈现相反趋势。皮下植入后,气泡在水凝胶中持续存在两周,导致局部胶原排列和核不对称增加。这些结果为以非侵入性和按需方式控制间叶干细胞的三维分化迈出了重要一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
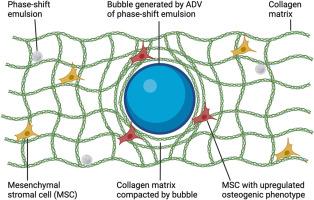
Ultrasound-generated bubbles enhance osteogenic differentiation of mesenchymal stromal cells in composite collagen hydrogels
Hydrogels can improve the delivery of mesenchymal stromal cells (MSCs) by providing crucial biophysical cues that mimic the extracellular matrix. The differentiation of MSCs is dependent on biophysical cues like stiffness and viscoelasticity, yet conventional hydrogels cannot be dynamically altered after fabrication and implantation to actively direct differentiation. We developed a composite hydrogel, consisting of type I collagen and phase-shift emulsion, where osteogenic differentiation of MSCs can be non-invasively modulated using ultrasound. When exposed to ultrasound, the emulsion within the hydrogel was non-thermally vaporized into bubbles, which locally compacted and stiffened the collagen matrix surrounding each bubble. Bubble growth and matrix compaction were correlated, with collagen regions proximal (i.e., ≤ ∼60 μm) to the bubble displaying a 2.5-fold increase in Young's modulus compared to distal regions (i.e., > ∼60 μm). The viability and proliferation of MSCs, which were encapsulated within the composite hydrogel, were not impacted by bubble formation. In vitro and in vivo studies revealed encapsulated MSCs exhibited significantly elevated levels of RUNX2 and osteocalcin, markers of osteogenic differentiation, in collagen regions proximal to the bubble compared to distal regions. Additionally, alkaline phosphatase activity and calcium deposition were enhanced adjacent to the bubble. An opposite trend was observed for CD90, a marker of MSC stemness. Following subcutaneous implantation, bubbles persisted in the hydrogels for two weeks, which led to localized collagen alignment and increases in nuclear asymmetry. These results are a significant step toward controlling the 3D differentiation of MSCs in a non-invasive and on-demand manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


