通过 Zn2+、Mn2+ 双掺杂改善 LiFe0.9Zn0.1-xMnxPO4(x=0,0.05,0.075,0.1)的电化学特性
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
通过简单的一步水热法合成了 Zn2+ 和 Mn2+ 共掺杂 LiFe0.9Zn0.1-xMnxPO4(其中 x = 0、0.05、0.075、0.1)复合材料。利用 XRD、SEM、TEM、EDS、CV 和交流阻抗测试对材料的组成、结构、形貌和电化学性能进行了全面表征。结果表明,Zn2+ 和 Mn2+ 的共掺杂不仅保留了 LiFePO4 的橄榄石结构,还稳定了晶体结构,降低了电荷转移电阻,提高了 Li+ 的扩散速率。当 Zn = 0.025 和 Mn = 0.075 掺杂比的正极材料的初始比容量为 161.8 mAh-g-1 时,200 次循环后的放电比容量仍高达 157.8 mAh-g-1,容量保持率为 97.5%,明显优于其他材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
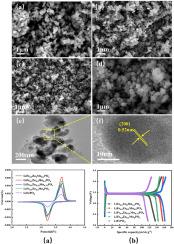
Improvement of electrochemical properties of LiFe0.9Zn0.1-xMnxPO4 (x=0, 0.05, 0.075, 0.1) by double doping with Zn2+, Mn2+
Zn2+ and Mn2+ co-doped LiFe0.9Zn0.1-xMnxPO4 (where x = 0, 0.05, 0.075, 0.1) composites are synthesized by a simple one-step hydrothermal method. The composition, structure, morphology and electrochemical properties of the materials are fully characterized using XRD, SEM, TEM, EDS, CV and AC impedance tests. The results show that the co-doping of Zn2+ and Mn2+ preserves the olivine structure of LiFePO4 and also stabilizes the crystal structure, reduces the charge transfer resistance and improves the Li+ diffusion rate. When the initial specific capacity of the cathode material with the doping ratio of Zn = 0.025 and Mn = 0.075 is 161.8 mAh·g−1, the discharge specific capacity after 200 cycles is still as high as 157.8 mAh·g−1 with a capacity retention of 97.5 %, which is obviously better than the rest of the materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


