通过修正的统计物理模型了解稻壳灰制备的混合沸石上二氧化碳捕获的吸附机理
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
在这项研究中,我们建立了一个基于统计物理学的改进型高级双层模型,并利用该模型探讨了二氧化碳(CO2)在两种沸石上的吸附情况:W-ZSM-5和W-硅铝酸盐-1。与其他只考虑一种化学势的双层模型不同,该模型的建立假设界面现象涉及不同的化学势。这种表述方式能更好地理解气体的多层吸附。这个新模型得出的结果表明,在 CO2-W-ZSM-5 系统中,二氧化碳分子改变了它们的吸附取向,从混合取向(n = 0.88)变为平行和非平行构型。同样,二氧化碳分子在 W 硅灰石-1 表面的吸附也从多分子取向(n = 1.17)转变为垂直取向。计算得出的吸附能证实,二氧化碳分子与这些沸石表面之间的物理相互作用控制着一个放热的吸附过程。最后,分析了两种吸附剂表面的吸附能分布 (AED),以确定二氧化碳捕获过程中激活的能带。这些发现有助于加深对沸石表面二氧化碳吸附的理解。这种新的统计物理模型可用于各种应用中气体吸附系统的工艺设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
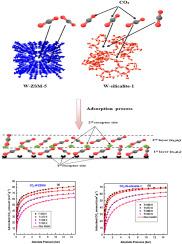
Understanding the adsorption mechanism of carbon dioxide capture on hybrid zeolites prepared from rice husk ash via a modified statistical physics model
In this study, a modified advanced double-layer model based on statistical physics was developed and utilized to explore the adsorption of carbon dioxide (CO2) on two zeolites: W-ZSM-5 and W-silicalite-1. This model was formulated assuming that different chemical potentials were involved in the interfacial phenomenon, in contrast to other double-layer models that consider only one chemical potential. This formulation provides a better understanding of the multilayer adsorption of gases. The results obtained from this new model indicate that in the case of the CO2-W-ZSM-5 system, CO2 molecules altered their adsorption orientation from a mixed orientation (n = 0.88) involving both parallel and non-parallel configurations. Similarly, the molecules shifted from a multimolecular orientation (n = 1.17) to a perpendicular orientation for CO2 adsorption on the W-silicalite-1 surface. The calculated adsorption energies confirmed the presence of an exothermic adsorption process governed by physical interactions between the CO2 molecules and the surfaces of these zeolites. Finally, the adsorption energy distribution (AED) of both adsorbent surfaces was analyzed to determine the energy band activated during the CO2 capture process. These findings contribute to a deeper understanding of CO2 adsorption on zeolite surfaces. This new statistical physics model can be used for the process design of gas adsorption systems in various applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


