5,5-双(4-氟苯基)咪唑烷-2,4-二酮(苯妥英的双(对氟苯基)衍生物)的理化性质和细胞色素 P-450 动力学
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
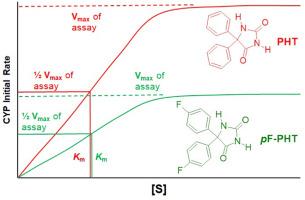
苯妥英(PHT,品牌名:Dilantin)是一种抗惊厥药物,用于治疗癫痫。PHT 通过细胞色素 P-450 (CYP) 催化芳香族对位羟基化作用而代谢失活。因此,Nelson 等人假设,芳香环对位含氟的 PHT 衍生物(pF-PHT)会减慢或阻断这种代谢途径,从而产生一种抗癫痫活性更强、作用时间比 PHT 更长的分子。有趣的是,pF-PHT 的活性低于 PHT,但作用时间却更长。Nelson 等人推测,pF-PHT 活性低的原因肯定是理化性质的差异。因此,他们合成了 pF-PHT,以比较其与 PHT 的理化性质。此外,由于 PHT 在肝脏中会被 CYP 代谢灭活,因此还使用 Sprague Dawley(SD)大鼠肝脏微粒体对 CYP 催化氧化的动力学进行了比较。之前报道的 pF-PHT 合成方法使用了剧毒试剂并产生了剧毒气体。因此,我们开发了一种更安全的 pF-PHT 合成路线。这种合成方法分为三个步骤:1) 对氟苯甲醛在硫胺催化下进行安息香缩合;2) 安息香产物在硝酸氧化下生成相应的苯偶姻衍生物;3) 苯偶姻衍生物与尿素在微波辅助下进行苯妥英合成。PHT 和 pF-PHT 在共轭、酸度和亲油性方面没有明显差异。因此,我们的研究结果并不支持 pF-PHT 活性低于 PHT 是由于理化性质差异造成的这一假设。虽然 PHT 和 pF-PHT 与 SD 大鼠肝脏微粒体的 CYP 蛋白组具有相同的表面结合亲和力,但 pF-PHT 在 CYP 催化下的氧化速度只有 PHT 的一半。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Physicochemical properties and cytochromes P-450 kinetics of 5,5-bis(4-fluorophenyl)imidazolidine-2,4-dione, the bis(para-fluorophenyl) derivative of phenytoin
Phenytoin (PHT, brand name: Dilantin) is an anticonvulsant drug that is used in the treatment of epilepsy. PHT is metabolically inactivated by cytochromes P-450 (CYP) catalyzed aromatic hydroxylation at the para position. Therefore, Nelson et al. hypothesized that this metabolic pathway would be slowed or blocked for a PHT derivative with fluorines at the para positions of the aromatic rings (pF-PHT) resulting in a molecule with increased antiseizure activity and longer duration of action relative to PHT. Interestingly, pF-PHT is less active than PHT, but has a much longer duration of action. Nelson et al. hypothesized that differences in physicochemical properties must contribute to the poor activity of pF-PHT. Thus, pF-PHT was synthesized to compare its physicochemical properties with those of PHT. In addition, the kinetics of CYP catalyzed oxidation were compared using Sprague Dawley (SD) rat liver microsomes because PHT is metabolically inactivated by CYP in the liver. The previously reported synthesis of pF-PHT employs a highly toxic reagent and produces a highly poisonous gas. Therefore, a safer synthetic route for pF-PHT was developed. This synthetic approach utilizes three steps: 1) a thiamine catalyzed benzoin condensation of para-fluorobenzaldehyde, 2) a nitric acid oxidation of the benzoin product to the corresponding benzil derivative, and 3) a microwave-assisted phenytoin synthesis of this benzil derivative with urea. There are no significant differences in conjugation, acidity, and lipophilicity between PHT and pF-PHT. Therefore, our results do not support the hypothesis that the low activity of pF-PHT relative to PHT results from variations in physicochemical properties. While PHT and pF-PHT have the same apparent binding affinity for the CYP proteome of the SD rat liver microsome, pF-PHT undergoes CYP catalyzed oxidation at half the rate in comparison to PHT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


