在分子动力学模拟的辅助下,简化奥希替尼的结构,开发出具有抗增殖和抗SARS-CoV-2活性的新型吲哚基嘧啶-5-甲腈衍生物
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
基于对药物奥希替尼的简化和改良,我们创造并合成了两个新系列的吲哚基嘧啶-5-甲腈支架,它们具有抗SARS-Cov-2和抗癌的双重作用。新合成的杂环化合物的化学结构得到了有效表征。化合物 9b、10 和 15 对 SARS-CoV-2 的 IC50 值分别为 18.52、20.89 和 19.85,而作为药理对照的雷米西韦和氯喹的 IC50 值分别为 3.38 μM 和 24.9 μM。此外,化合物 9a 和 13 对 HepG2 细胞株具有抗增殖活性,其 IC50 值分别为 5.63 μM 和 3.06 μM,而多柔比星的 IC50 值为 7.4 μM。qRT-PCR 显示,与多柔比星相比,用化合物 9a 和 13 处理的 HepG2 细胞显示 p53 表达水平升高,CDK1 和 PI3K 表达水平降低。对 PI3Kα、PI3Kγ、CDK 和活性化合物复合物进行了持续 20 ns 的分子动力学模拟。结果证实,如分子对接数据所示,化合物 13 有可能成为临床前和临床研究的候选治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
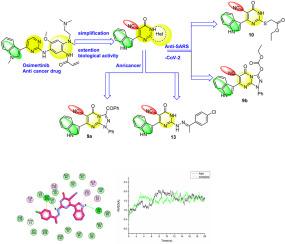
Structural simplification of Osimertinib to elaborate new indolyle-pyrimidine-5-carbonitrile derivatives with Anti-proliferative and Anti-SARS-CoV-2 activities assisted by molecular dynamic simulation
Based on the simplicity and modification of the medication osimertinib, two new series of indolyle-pyrimidine-5-carbonitrile scaffolds were created and synthesized with dual action as anti-SARS-Cov-2 and anticancer. The newly created heterocyclic compounds' chemical structures were effectively characterized. With IC50 values of 18.52, 20.89, and 19.85, respectively, compounds 9b, 10, and 15 had inhibitory actions against SARS-CoV-2 when compared to remdesivir and chloroquine, which served as pharmacological controls and had IC50 values of 3.38 μM and 24.9 μM, respectively.
Furthermore, compounds 9a and 13 showed anti-proliferative activity against HepG2 cell lines with IC50s of 5.63 μM and 3.06 μM, respectively, in comparison to doxorubicin's IC50 of 7.4 μM. qRT-PCR revealed that HepG2 cells treated with compounds 9a and 13 showed increased p53 expression levels and decreased CDK1 and PI3K expression levels in comparison to doxorubicin. Molecular dynamics simulations of 20 ns duration were performed with PI3Kα, PI3Kγ, and CDK and active compound complexes. The results confirm that compound 13 has the potential to be a therapeutic candidate for additional preclinical and clinical research, as indicated by the molecular docking data.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


