DMSO 可抑制 Talaromyces sp.(菌株 IQ-313)的杜仲苷生物合成途径,并解除萜类化合物、多酮类化合物和经萜类化合物的生物合成过程
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
Talaromyces sp.(菌株 IQ-313)在大米和燕麦谷物中的标准发酵条件下产生杜冷丁类分子。这类分子因其结构的复杂性和生物活性而备受关注。有趣的是,应用 OSMAC(一种菌株多种化合物)方法发现,这种真菌的生物合成机制可以定向用于生产其他类型的分子,同时完全抑制杜冷丁的生物合成(1)。为了开发塔拉酵母菌(菌株 IQ-313)的代谢潜力,研究人员在固体培养基中添加了二甲基亚砜(DMSO)(一种异位表观遗传调节剂)。利用分子网络对菌株在标准条件下和添加二甲基亚砜条件下的发酵提取物进行代谢组学分析,发现两者的代谢组学特征存在明显差异。通过对非生物胁迫条件下提取物的化学研究,分离并鉴定了 15 种在标准条件下未检测到的分子,包括 9 种多酮类化合物、1 种倍半萜类化合物、2 种酯萜类化合物和 3 种经萜类化合物。其中,talaromophilane (2)(一种eremophilane倍半萜类化合物)和talaroisochromane (8)(一种oxoisochromen类化合物)以前从未在文献中报道过。所有分离物的结构都是通过光谱和分光数据相结合的方法确定的。化合物 2 的绝对构型是根据 NOESY 相互作用的分析以及实验和理论电子圆二色性曲线的比较确定的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
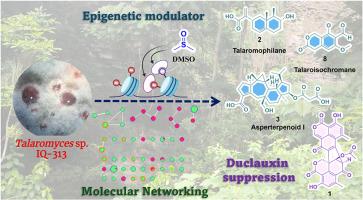
DMSO suppresses duclauxin biosynthetic pathway in Talaromyces sp. (strain IQ-313) and untaps terpenoids, polyketides and meroterpenoids biosynthesis
Talaromyces sp. (strain IQ-313) produces duclauxin-type molecules under standard fermentation conditions in rice and oat cereal. Such molecules are of great interest due to their structural complexity and biological activity. Interestingly, application of an OSMAC (One Strain Many Compounds) approach, revealed that the biosynthetic machinery of this fungus can be directed towards the production of other types of molecules, while completely suppressing the biosynthesis of duclauxin (1). To exploit the metabolic potential of Talaromyces sp. (strain IQ-313), it was grown on solid media supplemented with dimethyl sulfoxide (DMSO), an ectopic epigenetic modulator. Metabolomic analysis of the fermentation extract from the strain grown under standard and DMSO-supplemented conditions, using molecular networking, revealed notable differences in the profiles. Chemical investigation of the extract obtained under abiotic stress led to the isolation and characterization of 15 molecules not detected under standard conditions, including nine polyketides, one sesquiterpenoid, two sesterterpenoids, and three meroterpenoids. Among these, talaromophilane (2), an eremophilane sesquiterpenoid, and talaroisochromane (8), an oxoisochromen, have not been previously reported in the literature. The structures of all isolates were established using a combination of spectroscopic and spectrometric data. The absolute configuration of compound 2 was established based on the analysis of NOESY interactions and by comparison of the experimental and theoretical electronic circular dichroism (ECD) curves.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


