提高难溶性药物溶解度和口服生物利用度的制药方法。
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-09-21
DOI:10.1016/j.ejpb.2024.114513
引用次数: 0
摘要
在水和生理液体中的高溶解度是活性药物成分发挥药效不可或缺的条件。事实上,众所周知,在水中溶解度有限的药物在口服后会出现吸收减少和吸收不一致的情况,从而导致治疗效果的变化。目前,组合化学和药物设计的进步促进了以亲脂性增强、分子尺寸增大和水溶性降低为特征的候选药物的诞生。毫无疑问,近年来水溶性差的药物问题一直在逐步升级。事实上,在美国市场上销售的前 200 种口服药物中有 40%、33% 的药物被列入美国药典、75% 的在研化合物和 90% 的新化学实体都是水溶性不足的化合物。为了解决这一障碍,制剂科学家采用了多种方法,包括物理和化学方法,如原药合成、制盐、固体分散体的形成、亲水性物质的利用、增溶剂的加入、共溶剂的添加、多态性的探索、共晶体的创造、环糊精的复合、脂质制剂、粒度的减小和纳米制剂技术。尽管采用了这些不同的方法,但新药开发失败的主要原因仍然是药物化合物的水溶性差。因此,本文深入探讨了实施各种制剂策略的基本原则,并讨论了每种方法各自的优缺点。此外,本文还论述了在选择合适的制剂策略方面做出明智决策的方法框架,以有效应对开发水溶性差的候选药物过程中面临的关键挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
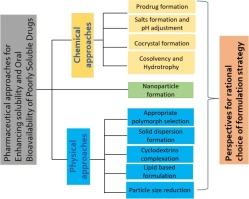
Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs
High solubility in water and physiological fluids is an indispensable requirement for the pharmacological efficacy of an active pharmaceutical ingredient. Indeed, it is well established that pharmaceutical substances exhibiting limited solubility in water are inclined towards diminished and inconsistent absorption following oral administration, consequently resulting in variability in therapeutic outcomes. The current advancements in combinatorial chemistry and pharmaceutical design have facilitated the creation of drug candidates characterized by increased lipophilicity, elevated molecular size, and reduced aqueous solubility. Undoubtedly, the issue of poorly water-soluble medications has been progressively escalating over recent years. Indeed, 40% of the top 200 oral medications marketed in the United States, 33% of drugs listed in the US pharmacopoeia, 75% of compounds under development and 90% of new chemical entities are insufficiently water-soluble compounds. In order to address this obstacle, formulation scientists employ a variety of approaches, encompassing both physical and chemical methods such as prodrug synthesis, salt formation, solid dispersions formation, hydrotropic substances utilization, solubilizing agents incorporation, cosolvent addition, polymorphism exploration, cocrystal creation, cyclodextrins complexation, lipid formulations, particle size reduction and nanoformulation techniques. Despite the utilization of these diverse approaches, the primary reason for the failure in new drug development persists as the poor aqueous solubility of pharmaceutical compounds. This paper, therefore, delves into the foundational principles that underpin the implementation of various formulation strategies, along with a discussion on the respective advantages and drawbacks associated with each approach. Additionally, a discourse is provided regarding methodological frameworks for making informed decisions on selecting an appropriate formulation strategy to effectively tackle the key challenges posed during the development of a poorly water-soluble drug candidate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


