纹状体细胞中 SREBP2 的减少与亨廷顿氏病的蛋白质降解紊乱有关。
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
这项研究揭示了胆固醇代谢、蛋白质降解机制与亨廷顿氏病(HD)发病机制之间错综复杂的关系。通过使用动物模型和细胞系统进行研究,我们观察到胆固醇水平发生了显著变化,尤其是在纹状体中,而纹状体是 HD 的主要病变部位。我们的研究结果表明,胆固醇代谢相关因子(如 LDLR 和 SREBP2)在 HD 中的失调可能会导致疾病进展。此外,我们还发现了蛋白质降解途径的紊乱,包括内切酶蛋白减少和自噬失调,这进一步加剧了HD的病理变化。此外,我们的研究还强调了针对这些途径的潜在治疗意义。通过恢复胆固醇水平和调节蛋白质降解机制,特别是通过 MLN4924 等干预措施,我们观察到细胞功能的潜在改善,BDNF 水平的提高就表明了这一点。这些见解强调了同时解决胆固醇代谢和蛋白质降解问题以缓解 HD 病理学的重要性。总之,这项研究为我们提供了一个基本认识,即在 HD 和 HD 细胞中,SREBP2 的减少与胆固醇代谢紊乱导致的蛋白质降解系统功能失调之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
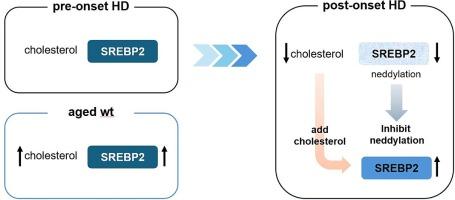
Decreased SREBP2 of the striatal cell relates to disrupted protein degradation in Huntington’s disease
This study delineated the intricate relation between cholesterol metabolism, protein degradation mechanisms, and the pathogenesis of Huntington’s disease (HD). Through investigations using both animal models and cellular systems, we have observed significant alterations in cholesterol levels, particularly in the striatum, which is the primary lesion site in HD. Our findings indicate the dysregulation of cholesterol metabolism-related factors, such as LDLR and SREBP2, in HD, which may contribute to disease progression. Additionally, we uncovered disruptions in protein degradation pathways, including decreased neddylated proteins and dysregulated autophagy, which further exacerbated HD pathology. Moreover, our study highlighted the potential therapeutic implications of targeting these pathways. By restoring cholesterol levels and modulating protein degradation mechanisms, particularly through interventions, such as MLN4924, we observed potential improvements in cellular function, as indicated by the increased BDNF levels. These insights underscore the importance of simultaneously addressing cholesterol metabolism and protein degradation to alleviate HD pathology. Collectively, this study provides a basic understanding of the interplay between the decrease of SREBP2 and the dysfunctional protein degradation system derived from disrupted cholesterol metabolism in HD and HD cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


