SHIN-2 通过稳定丝氨酸羟甲基转移酶的活性位点环路,在体外对耐 VanA 型万古霉素的粪肠球菌发挥强效活性。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
需要新型抗生素来提高医疗保健系统对抗菌素耐药性(AMR)(包括万古霉素耐药性)的抵抗力。该基因簇可将万古霉素耐药性从肠球菌转移到金黄色葡萄球菌。因此,需要新型抗菌剂来对抗 AMR,包括 VanA 型万古霉素耐药性。丝氨酸羟甲基转移酶(SHMT)是抗菌药的一个关键靶点。然而,SHMT 抑制剂的具体结合机制仍不清楚。详细的结构信息将有助于了解这些机制。本研究发现,(+)-SHIN-2 是人类 SHMT 的首个体内活性抑制剂,能与粪肠球菌 SHMT(efmSHMT)强结合。通过比较apo-和(+)-SHIN-2-boud efmSHMT的晶体结构发现,(+)-SHIN-2通过氢键稳定了efmSHMT的活性位点环,这对efmSHMT的抑制作用至关重要。此外,(+)-SHIN-2 还与丝氨酸形成氢键,与作为 SHMT 辅因子的 5'- 磷酸吡哆醛形成希夫碱。此外,(+)-SHIN-2 还能在体外对万古霉素敏感和万古霉素耐药的粪肠球菌产生生物静电效应,表明 SHMT 抑制剂不会诱发对万古霉素的交叉耐药性。总之,这些发现有助于设计新型 SHMT 抑制剂,以对抗包括万古霉素耐药性在内的 AMR。本文章由计算机程序翻译,如有差异,请以英文原文为准。
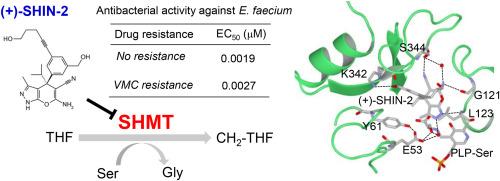
SHIN-2 exerts potent activity against VanA-type vancomycin-resistant Enterococcus faecium in vitro by stabilizing the active site loop of serine hydroxymethyltransferase
Novel classes of antibiotics are needed to improve the resilience of the healthcare system to antimicrobial resistance (AMR), including vancomycin resistance. vanA gene cluster is a cause of vancomycin resistance. This gene cluster is transferred and spreads vancomycin resistance from Enterococcus spp. to Staphylococcus aureus. Therefore, novel antibacterial agents are required to combat AMR, including vanA-type vancomycin resistance. Serine hydroxymethyltransferase (SHMT) is a key target of antibacterial agents. However, the specific binding mechanisms of SHMT inhibitors remain unclear. Detailed structural information will contribute to understanding these mechanisms. In this study, we found that (+)–SHIN–2, the first in vivo active inhibitor of human SHMT, is strongly bound to the Enterococcus faecium SHMT (efmSHMT). Comparison of the crystal structures of apo- and (+)–SHIN–2-boud efmSHMT revealed that (+)–SHIN–2 stabilized the active site loop of efmSHMT via hydrogen bonds, which are critical for efmSHMT inhibition. Additionally, (+)–SHIN–2 formed hydrogen bonds with serine, forming the Schiff's base with pyridoxal 5′-phosphate, which is a co-factor of SHMT. Furthermore, (+)–SHIN–2 exerted biostatic effects on vancomycin-susceptible and vanA-type vancomycin-resistant E. faecium in vitro, indicating that SHMT inhibitors do not induce cross-resistance to vanA-type vancomycin. Overall, these findings can aid in the design of novel SHMT inhibitors to combat AMR, including vancomycin resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


