醛糖还原酶与喹诺酮类抗生素的相互作用:体外和硅学方法研究其与糖尿病并发症的关系。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
醛糖还原酶(AR,EC1.1.1.21)是醛酮还原酶家族的成员之一,与糖尿病相关慢性并发症(包括神经病变、肾病变和视网膜病变)的发病机制密切相关。高血糖诱导的 AR 过度活跃会导致细胞内山梨醇积聚、NADPH 耗竭和氧化应激。因此,AR 被认为是氧化和炎症信号通路的关键介质,涉及心血管疾病、炎症性疾病和癌症等多种人类病症。这再次激发了人们对开发新型 AR 抑制剂(ARIs)的兴趣。在本研究中,我们评估了五种喹诺酮类抗生素--加替沙星、洛美沙星、萘啶酸、诺氟沙星和司帕沙星--作为与各种生理和病理条件相关的 ARIs 的抑制潜力。通过全面的体外和硅学分析,我们探索了这些抗生素与 AR 活性位点的结合相互作用和亲和力。我们的研究结果表明,这些喹诺酮类药物在微摩尔浓度下对AR有中度抑制作用,抑制常数(KIs)从1.03 ± 0.13 μM到4.12 ± 0.51 μM不等,而参比药物依帕司他(KI为0.85 ± 0.06 μM)的抑制常数为0.85 ± 0.06 μM。综合体外研究和硅学研究结果,这些药物与AR之间的相互作用非常明显,表明它们有可能成为治疗上述病症的药物。此外,这些见解将有助于优化临床用药方案,并在这些抗生素与其他疗法联合用药时减少意外的药物相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
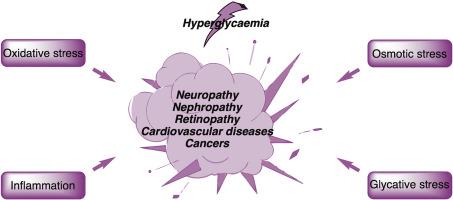
Aldose reductase with quinolone antibiotics interaction: In vitro and in silico approach of its relationship with diabetic complications
Aldose reductase (AR, EC1.1.1.21), a member of the aldo-keto reductase family, is critically implicated in the pathogenesis of chronic complications associated with diabetes mellitus, including neuropathy, nephropathy, and retinopathy. Hyperglycemia-induced AR overactivity results in intracellular sorbitol accumulation, NADPH depletion, and oxidative stress. Consequently, AR is recognized as a key mediator of oxidative and inflammatory signaling pathways involved in diverse human pathologies such as cardiovascular diseases, inflammatory disorders, and cancer. This has sparked renewed interest in developing novel AR inhibitors (ARIs) with enhanced therapeutic profiles. In this study, we evaluated the inhibitory potential of five quinolone antibiotics-gatifloxacin, lomefloxacin, nalidixic acid, norfloxacin, and sparfloxacin-as ARIs relevant to various physiological and pathological conditions. Through comprehensive in vitro and in silico analyses, we explored these antibiotics' binding interactions and affinities within the AR active site. Our findings reveal that these quinolones moderately inhibit AR at micromolar concentrations, with inhibition constants (KIs) ranging from 1.03 ± 0.13 μM to 4.12 ± 0.51 μM, compared to the reference drug epalrestat (KI of 0.85 ± 0.06 μM). The combined in vitro and in silico results underscore significant interactions between these drugs and AR, suggesting their potential as therapeutic agents against the aforementioned pathological conditions. Furthermore, these insights will aid in optimizing clinical dosing regimens and mitigating unexpected drug-drug interactions when these antibiotics are co-administered with other treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


