COVID-19 时代冠状动脉粥样硬化和不明原因猝死的免疫学研究。
IF 9.2
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
包括 SARS-CoV-2 感染在内的病毒感染导致心脏死亡的免疫学基础仍然是个谜。婴儿川崎病(KD)和儿童多系统炎症综合征(MIS-C)中伴有冠状动脉血管炎的心外膜炎症与冠状动脉血管炎有关。在这篇视角独特的文章中,我们回顾了与病毒感染相关的老年心外膜炎症的证据,在老年心外膜炎症中,动脉粥样硬化斑块失稳与看似无关的心肌梗死可能与实际的感染诱因无关。冠状动脉穿过的心外膜组织中的心外膜心肌炎在慢性阻塞性肺疾病后的成人气肿期很常见,在接种疫苗(包括 COVID 时代前的疫苗接种)后也有大量描述。从免疫学角度看,在 COVID-19 时代之前,心外膜组织对冠状动脉区域动脉粥样硬化性疾病至关重要。我们强调了与病毒感染或疫苗接种相关的弥漫性心外膜组织间质炎症是如何在邻近冠状动脉血管区域代表了一个重要的统一概念,即看似无关的致命性冠状动脉粥样硬化疾病与成人感染或疫苗接种无关。从机理上讲,这种影响冠状动脉血管免疫的心外膜炎症是预先存在的动脉粥样硬化斑块缓慢失稳的网关,会导致心肌梗塞和其他心脏病变。这一模型为免疫学家和心脏病学家提供了一个免疫病理路线图,它介于无害的病毒感染或疫苗接种与看似时间遥远的 "无关 "动脉粥样硬化疾病和过多的心脏病死亡之间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
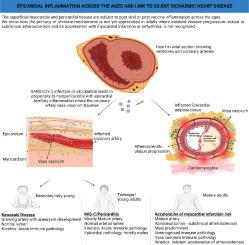
An immunology model for accelerated coronary atherosclerosis and unexplained sudden death in the COVID-19 era
The immunological basis for cardiac deaths remote from potential triggering viral infection, including SARS-CoV-2 infection, remains enigmatic. Cardiac surface inflammation, including the pericardium, epicardium and superficial myocardium with associated coronary artery vasculitis in infant Kawasaki Disease (KD) and multisystem inflammatory syndrome in children (MIS-C) is well recognised. In this perspective, we review the evidence pointing towards prominent post-viral infection related epicardial inflammation in older subjects, resulting in atherosclerotic plaque destabilisation with seemingly unrelated myocardial infarction that may be temporally distant from the actual infectious triggers. Cardiac surface inflammation in the relatively immune cell rich tissues in the territory though where the coronary arteries traverse is common in the adult post-COVD pneumonic phase and is also well described after vaccination including pre-COVID era vaccinations. Immunologically, the pericardium/epicardium tissue was known to be critical for coronary artery territory atherosclerotic disease prior to the COVID-19 era and may be linked to the involvement of the coronary artery vasa vasorum that physiologically oxygenates the coronary artery walls. We highlight how viral infection or vaccination-associated diffuse epicardial tissue inflammation adjacent to the coronary artery vasa vasorum territory represents a critical unifying concept for seemingly unrelated fatal coronary artery atherosclerotic disease, that could occur soon after or remote from infection or vaccination in adults. Mechanistically, such epicardial inflammation impacting coronary artery vasa vasorum immunity acts as gateways towards the slow destabilisation of pre-existing atherosclerotic plaques, with resultant myocardial infarction and other cardiac pathology. This model offers immunologists and academic cardiologists an immunopathological roadmap between innocuous viral infections or vaccinations and seemingly temporally remote “unrelated” atherosclerotic disease with excess cardiac deaths.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


