通过反相乳液的 pH 值诱导油相转变实现减阻剂的快速释放
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
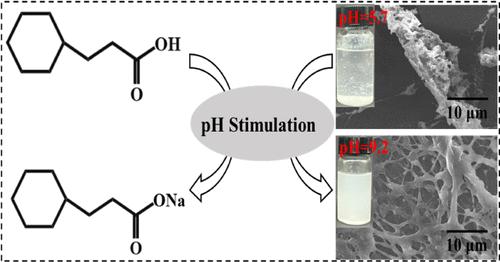
作为滑油压裂液的关键成分,减阻乳化液起着至关重要的作用。传统的降乳剂通过添加亲水性表面活性剂来提高溶解能力,这导致降乳剂的稳定性较差。为了消除乳液减阻剂稳定性和释放性之间的矛盾,本文以切换溶剂(HA)和白油为连续相,制备了 pH 响应聚合物乳液。单体乳液表现出明显的 pH 响应行为。这是因为在 pH 值的刺激下,HA 的去质子化会导致乳液的油水比发生变化,从而促进乳液的破乳化。单体乳液的显著稳定性有利于制备反相乳液聚合物(P(AM-AA-AMPS))。获得的 P(AM-AA-AMPS)聚合物乳液在储存 15 天后仍具有显著的稳定性。重要的是,通过 pH 值刺激,P(AMA-AMPS) 聚合物能从乳液中有效释放,而不是引入额外的亲水性表面活性剂。在 pH 值为 9.2 时,P(AMA-AMPS) 聚合物水溶液的粘度在 80 秒内达到最大值 96 mPa s。与传统聚合物乳液(2 分钟)相比,P(AA-AMA-AMPS) 聚合物乳液的释放效率提高了 33%。P(AM-AA-AMPS) 乳液具有显著的阻力降低性能,当浓度为 0.05 wt % 时,阻力降低率达到 73%。具有 pH 响应性的 P(AMA-AMPS)聚合物乳液消除了乳液减阻剂稳定性与释放之间的矛盾。基于 pH 响应型 P(AMA-AMPS)聚合物乳液的研究为开发快速溶解和长期储存的降阻剂提供了其他思路,有助于低渗透油气资源的开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Realizing the Rapid Release of Drag Reducers via pH-Induced Oil Phase Transition of Inverse Emulsion
As a key component of slickwater fracturing fluids, emulsion drag reducers play a vital role. The dissolving capacity of traditional emulsion drag reducers is improved by adding hydrophilic surfactants, which leads to poor stability of the emulsion drag reducer. In order to eliminate the contradiction between stability and release of the emulsion drag reducer, here, pH-responsive polymer emulsion was fabricated using the switching solvent (HA) and white oil as the continuous phase. Monomer emulsions exhibit obvious pH-responsive behavior. This is because the deprotonation of HA by pH stimulation leads to a change in the oil–water ratio of the emulsion, thereby facilitating the demulsification of emulsion. The remarkable stability of the monomer emulsion benefits the preparation of inverse emulsion polymers (P(AM-AA-AMPS)). The obtained P(AM-AA-AMPS) polymer emulsion features remarkable stability even after 15 days of storage. Importantly, the P(AM-AA-AMPS) polymer was released from the emulsion efficiently by pH stimulation instead of introducing an extra hydrophilic surfactant, which confirmed the improvement of polymer release by pH stimulation. The viscosity of the P(AM-AA-AMPS) polymer aqueous solution reaches a maximum value of 96 mPa s within 80 s at a pH value of 9.2. The release efficiency of P(AA-AM-AMPS) polymer emulsion is increased by 33% in comparison with that of traditional polymer emulsion (2 min). The P(AM-AA-AMPS) emulsion demonstrated remarkable drag-reduction performance by achieving a drag-reduction rate of 73% at a concentration of 0.05 wt %. P(AM-AA-AMPS) polymer emulsion with pH responsiveness eliminates the contradiction between the stability and release of emulsion drag reducers. Research based on pH-responsive P(AM-AA-AMPS) polymer emulsion provides other ideas for the development of quickly dissolving and long-term storage drag reducers, which is helpful for the development of low-permeability oil and gas resources.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


