利用新颖的定点酶强化疗法(SEE-Tx)药物发现平台确定治疗 1 型戊二酸血症的药理合剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
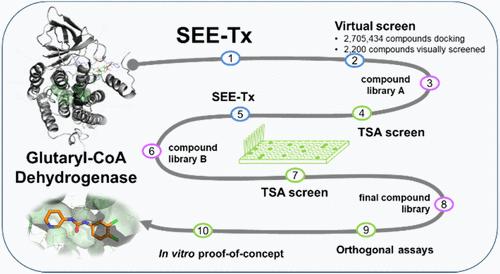
作为药理伴侣的异构调节剂具有创新疗法的前景,因为它们靶向非催化位点,在不与天然底物竞争的情况下稳定折叠蛋白,从而实现功能净增。外源性异构调节剂通常比活性位点抑制剂更具选择性,当天然底物水平较高时,外源性异构调节剂可能比竞争性抑制剂更有效。为了鉴定能与线粒体酶戊二酰-CoA脱氢酶(GCDH)结合并使其稳定的新型结构靶向异位调节剂(STARs),我们应用了计算位点定向酶增强疗法(SEE-Tx)技术。SEE-Tx是一种创新的药物发现平台,有望发现治疗蛋白质错误折叠疾病(如戊二酸血症1型(GA1))的药物。利用基于结构和配体的虚拟筛选方法发现了推定的异位调节剂,并利用正交的生物物理和生物化学实验进行了验证。本文介绍的计算方法可用于发现其他蛋白质错误折叠疾病的异构调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Use of the Novel Site-Directed Enzyme Enhancement Therapy (SEE-Tx) Drug Discovery Platform to Identify Pharmacological Chaperones for Glutaric Acidemia Type 1
Allosteric regulators acting as pharmacological chaperones hold promise for innovative therapeutics since they target noncatalytic sites and stabilize the folded protein without competing with the natural substrate, resulting in a net gain of function. Exogenous allosteric regulators are typically more selective than active site inhibitors and can be more potent than competitive inhibitors when the natural substrate levels are high. To identify novel structure-targeted allosteric regulators (STARs) that bind to and stabilize the mitochondrial enzyme glutaryl-CoA dehydrogenase (GCDH), the computational site-directed enzyme enhancement therapy (SEE-Tx) technology was applied. SEE-Tx is an innovative drug discovery platform with the potential to identify drugs for treating protein misfolding disorders, such as glutaric acidemia type 1 (GA1) disease. Putative allosteric regulators were discovered using structure- and ligand-based virtual screening methods and validated using orthogonal biophysical and biochemical assays. The computational approach presented here could be used to discover allosteric regulators of other protein misfolding disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


