氢释放镁水凝胶通过抑制中性粒细胞外捕获物减轻椎板切除术后硬膜外纤维化。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
硬膜外纤维化是导致椎板切除手术失败的主要原因,从而引发背部手术失败综合征(FBSS)。椎板切除术后,中性粒细胞浸润手术部位,产生中性粒细胞胞外陷阱(NET),导致硬膜外纤维化。活性氧(ROS)在介导 NETs 的形成中起着关键作用。分子氢因其选择性抗氧化特性和生物安全性而成为抑制硬膜外纤维化的潜在治疗气体。在这项研究中,我们开发了一种原位氢释放水凝胶,它能抑制 NET 的形成并减轻硬膜外瘢痕。可生物降解的镁(Mg)微球作为氢源,外覆聚乳酸(PLGA)以调节氢的释放。然后将这些微球(Mg@PLGA)与 PLGA-PEG-PLGA 热敏水凝胶(Mg@PLGA@Gel)结合在一起,提供了一种可长期持续释放氢气的外科植入物。体外实验证实了该系统的生物相容性,证明 Mg@PLGA 产生的氢气能有效中和中性粒细胞内的 ROS 并抑制 NETs 的形成。组织学分析,包括 H&E 染色、核磁共振成像、Masson 染色和免疫组化,共同表明 Mg@PLGA@Gel 具有生物相容性,能有效抑制椎板切除术后硬膜外纤维化。此外,Mg@PLGA@Gel 还能抑制手术部位的 ROS 积累和 NET 的形成。这些研究结果表明,Mg@PLGA@Gel 可确保持续的治疗性氢浓度,从而缓解椎板切除术小鼠模型的硬膜外纤维化。意义声明-释放氢气的水凝胶结合了物理屏障和免疫调节的治疗效果。-原位生成的分子氢能清除手术应激引起的 ROS 并抑制 NETs 的形成。-氢释放水凝胶具有很高的生物相容性,可抑制体内硬膜外瘢痕的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
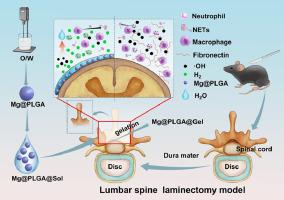
Hydrogen-releasing magnesium hydrogel mitigates post laminectomy epidural fibrosis through inhibition of neutrophil extracellular traps
Epidural fibrosis is a primary contributor to the failure of laminectomy surgeries, leading to the development of failed back surgery syndrome (FBSS). Post-laminectomy, neutrophils infiltrate the surgical site, generating neutrophil extracellular traps (NETs) that contribute to epidural fibrosis. Reactive oxygen species (ROS) play a pivotal role in mediating NETs formation. Molecular hydrogen, recognized for its selective antioxidant properties and biosafety, emerges as a potential therapeutic gas in suppressing epidural fibrosis. In this study, we developed an in-situ hydrogen release hydrogel that inhibits the formation of NETs and mitigates epidural scarring. Biodegradable magnesium (Mg) microspheres served as a hydrogen source, coated with PLGA to regulate hydrogen release. These microspheres (Mg@PLGA) were then incorporated into a PLGA-PEG-PLGA thermosensitive hydrogel (Mg@PLGA@Gel), providing a surgical implant for sustained, long-term hydrogen release. In vitro experiments confirmed the biocompatibility of the system, demonstrating that hydrogen produced by Mg@PLGA effectively neutralizes neutrophil intracellular ROS and inhibits NETs formation. Histological analyses, including H&E staining, MRI, Masson staining, and immunohistochemistry, collectively indicate that Mg@PLGA@Gel is biocompatible and effectively inhibits epidural fibrosis post-laminectomy. Furthermore, Mg@PLGA@Gel inhibits ROS accumulation and NETs formation at the surgical site. These findings suggest that Mg@PLGA@Gel ensures continuous, therapeutic hydrogen concentration, providing relief from epidural fibrosis in a laminectomy mouse model.
Statement of significance
- •The hydrogen-releasing hydrogel combines the therapeutic effects of a physical barrier with immunomodulation.
- •In situ-generated molecular hydrogen scavenges ROS caused by surgical stress and suppresses NETs formation.
- •The hydrogen-releasing hydrogel is demonstrated to exhibit high biocompatibility and inhibit epidural scar formation in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


