免疫调节巨噬细胞的收养性转移可降低促炎微环境并增加钛植入物上的骨形成。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
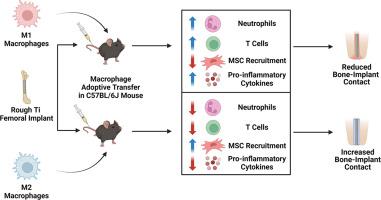
巨噬细胞在协调对植入生物材料的炎症反应中发挥着核心作用,并对植入物的化学和物理特性变化十分敏感。巨噬细胞对生物、化学和物理线索的反应是极化为促炎(M1)或抗炎(M2)状态。我们以前的研究表明,粗糙亲水性钛(Ti)植入物会使巨噬细胞向抗炎表型极化,并增加间充质干细胞(MSC)的招募和植入物周围的骨形成。在本研究中,我们旨在探讨不同极化状态的巨噬细胞的收养性转移是否会改变炎症微环境,并改善巨噬细胞功能健全和巨噬细胞功能缺失小鼠的生物材料整合。我们发现,消融巨噬细胞会增加中性粒细胞的存在,减少 T 细胞和间充质干细胞,并损害愈合和生物材料整合过程。这些影响无法通过收养性转移天真或极化巨噬细胞得到挽救。将 M1 巨噬细胞收养性转移到具有巨噬细胞能力的小鼠体内会增加炎症细胞和炎症微环境,导致骨与种植体接触减少。将 M2 巨噬细胞收养转移到巨噬细胞功能正常的小鼠体内可减少种植体周围组织的促炎环境,增加骨与种植体的接触。综上所述,我们的研究结果表明了巨噬细胞在控制和调节植入生物材料的炎症反应过程中的重要性,并表明巨噬细胞可用于改善生物材料植入后的治疗效果。意义说明:巨噬细胞是协调植入生物材料炎症反应的核心,对生物材料的化学和物理特性非常敏感。我们的研究表明,巨噬细胞的缺乏会导致炎症持续时间延长,并破坏骨与生物材料的整合。将具有免疫调节功能的巨噬细胞收养转移到具有巨噬细胞功能的小鼠体内可减轻炎症环境并增加骨与植入物的接触。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adoptive transfer of immunomodulatory macrophages reduces the pro-inflammatory microenvironment and increases bone formation on titanium implants
Macrophages play a central role in orchestrating the inflammatory response to implanted biomaterials and are sensitive to changes in the chemical and physical characteristics of the implant. Macrophages respond to biological, chemical, and physical cues by polarizing into pro-inflammatory (M1) or anti-inflammatory (M2) states. We previously showed that rough-hydrophilic titanium (Ti) implants skew macrophage polarization towards an anti-inflammatory phenotype and increase mesenchymal stem cell (MSC) recruitment and bone formation around the implant. In the present study, we aimed to investigate whether the adoptive transfer of macrophages in different polarization states would alter the inflammatory microenvironment and improve biomaterial integration in macrophage-competent and macrophage-ablated mice. We found that ablating macrophages increased the presence of neutrophils, reduced T cells and MSCs, and compromised the healing and biomaterial integration process. These effects could not be rescued with adoptive transfer of naïve or polarized macrophages. Adoptive transfer of M1 macrophages into macrophage-competent mice increased inflammatory cells and inflammatory microenvironment, resulting in decreased bone-to-implant contact. Adoptive transfer of M2 macrophages into macrophage-competent mice reduced the pro-inflammatory environment in the peri‑implant tissue and increased bone-to-implant contact. Taken together, our results show the importance of macrophages in controlling and modulating the inflammatory process in response to implanted biomaterials and suggest they can be used to improve outcomes following biomaterial implantation.
Statement of significance
Macrophages are central in orchestrating the inflammatory response to implanted biomaterials and are sensitive to biomaterial chemical and physical characteristics. Our study shows that a deficiency of macrophages results in prolonged inflammation and abolishes bone-biomaterial integration. Adoptive transfer of immunomodulatory macrophages into macrophage-competent mice reduced the inflammatory environment and increased bone-implant contact.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


