用于加速生物膜感染慢性伤口愈合的光调节一氧化氮输送水凝胶。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
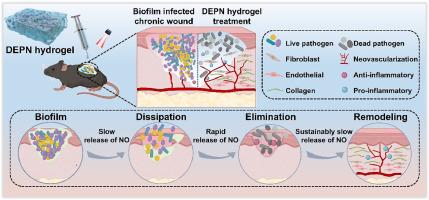
生物膜感染和慢性伤口愈合障碍给临床实践带来了巨大挑战。在这项研究中,我们提出了一种多功能抗菌水凝胶,它能以可控方式输送一氧化氮(NO),以消散生物膜、清除微生物并促进慢性伤口愈合。这种水凝胶是由氧化葡聚糖和抗菌肽ε-聚赖氨酸通过席夫碱交联构建而成,并进一步包裹了含有一氧化氮供体的光热纳米粒子。这种水凝胶能持续、缓慢地释放 NO,有效驱散生物膜,促进小鼠成纤维细胞的增殖和内皮细胞的迁移。在近红外激光照射下,水凝胶产生高热并迅速释放 NO,通过 NO、光热疗法和抗菌肽的协同作用,有效消除了多种耐药革兰氏阳性/阴性细菌和真菌生物膜。值得注意的是,这种水凝胶通过成功消除生物膜感染、调节炎症、促进血管生成和胶原蛋白沉积,在加速感染了耐甲氧西林金黄色葡萄球菌的小鼠糖尿病伤口愈合过程中显示出卓越的体内治疗效果。总之,这种拟议的水凝胶在适应受生物膜感染的慢性伤口复杂修复过程的各种需求方面显示出巨大的前景。意义说明生物膜感染的存在和愈合过程中的潜在功能障碍使慢性伤口陷入炎症阶段,难以愈合。这项研究开发了一种近红外激光调制的三级氮释放多功能抗菌水凝胶(DEPN),对慢性伤口具有良好的治疗效果。这种 DEPN 水凝胶本身可缓慢释放 NO 以驱散生物膜。在近红外激光照射下,DEPN 水凝胶会产生高热,并诱导 NO 快速爆发释放,从而有效消除多种耐药细菌和真菌生物膜。随后,DEPN 水凝胶继续缓慢释放 NO,促进组织重塑。这种 DEPN 水凝胶在治疗受生物膜感染的慢性伤口方面显示出巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A photo-modulated nitric oxide delivering hydrogel for the accelerated healing of biofilm infected chronic wounds
Biofilm infection and impaired healing of chronic wounds are posing tremendous challenges in clinical practice. In this study, we presented a versatile antimicrobial hydrogel capable of delivering nitric oxide (NO) in a controllable manner to dissipate biofilms, eliminate microorganisms, and promote the healing of chronic wounds. This hydrogel was constructed by Schiff-base crosslinking of oxidized dextran and antimicrobial peptide ε-poly-lysine, further encapsulating photothermal nanoparticles bearing NO donor. This hydrogel could continuously and slowly release NO, effectively dissipating biofilms, and promoting the proliferation of mouse fibroblasts and the migration of endothelial cells. Upon exposure to NIR laser irradiation, the hydrogel generated hyperthermia and rapidly released NO, resulting in the efficient elimination of a broad spectrum of drug-resistant Gram-positive/negative bacterial and fungal biofilms through the synergistic effects of NO, photothermal therapy, and the antibacterial peptide. Notably, the hydrogel demonstrated exceptional in vivo therapeutic outcomes in accelerating the healing process of mice diabetic wounds infected with methicillin-resistant Staphylococcus aureus by successfully eliminating biofilm infection, regulating inflammation, and facilitating angiogenesis and collagen deposition. Overall, this proposed hydrogel shows great promise in accommodating the various demands of the complex repair process of chronic wounds infected with biofilms.
Statement of significance
The presence of biofilm infections and underlying dysfunctions in the healing process made chronic wound become stuck in the inflammation stage and difficult to heal. This work developed a NIR laser-modulated three-stage NO-releasing versatile antimicrobial hydrogel (DEPN) exhibiting good therapeutic efficacy for chronic wound. This DEPN hydrogel could inherently and slowly released NO to disperse biofilm. Upon NIR laser irradiation, the DEPN hydrogel generated hyperthermia and induced a rapid burst release of NO effectively eliminating a broad spectrum of drug-resistant bacterial and fungal biofilms. Subsequently, the DEPN hydrogel continually release NO slowly to promote the tissue remolding. This DEPN hydrogel displays great potential in treatment of chronic wounds infected with biofilm.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


