基于Au-Cu2-xSe@ZIF-8的双途径猝灭诱导剂通过破坏锌离子平衡增强肿瘤免疫疗法
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
调节细胞内离子平衡以触发抗原特异性免疫反应在肿瘤治疗中引起了广泛关注。在这项研究中,我们开发了一种双途径纳米反应器--Au-Cu2-xSe@ZIF-8@P18 NPs(ACS-Z-P NPs),它以危险相关分子模式(DAMPs)为靶点,在肿瘤微环境(TME)中释放 Zn2+ 和活性氧(ROS)。从金属有机框架(MOFs)中释放出的 Zn2+ 沉积在细胞质中,导致细胞内锌调控蛋白的转录水平异常和 DNA 损伤,从而诱导依赖于 caspase1/gasdermin D(GSDMD)途径的热凋亡和免疫原性细胞死亡(ICD)。此外,在激光照射下,ACS-Z-P NPs 还能突破 TME 免疫抑制固有缺陷的限制,通过 Fenton 类级联反应增强 ROS 的生成,进而引发炎症小泡的活化和损伤相关分子模式(DAMPs)的释放。这种级联效应导致了热凋亡和免疫原性细胞死亡(ICD)的扩大,从而重塑了免疫抑制的 TME。因此,这一过程改善了树突状细胞(DC)的抗原呈递,增强了抗肿瘤 T 细胞反应,有效地启动了抗原特异性免疫反应,并进一步增强了热解和 ICD。本研究详细探讨了这些机制的治疗特性。重要意义合成的 Au-Cu2-xSe@ZIF-8@P18 纳米粒子(ACS-Z-Ps)可通过调节细胞内锌离子水平有效增强机体免疫反应。这种调节会导致锌调控蛋白转录水平异常和 DNA 损伤,从而诱发细胞的嗜热症。因此,树突状细胞(DC)的抗原呈递得到改善,抗肿瘤 T 细胞反应得到增强。ACS-Z-P NP 通过类似芬顿的反应利用肿瘤微环境中的 H2O2,克服了肿瘤微环境中 ROS 缺乏和免疫抑制的局限性。这导致了 ROS 和 O2 生成的增加、免疫抑制肿瘤微环境的重塑以及细胞热解和免疫原性细胞死亡诱导的增强。ACS-Z-P NPs 使用光敏剂 P18 靶向 B16 细胞,并结合 PDT 治疗。这种方法明显抑制了 B16 细胞的增殖,并有效抑制了肿瘤的生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
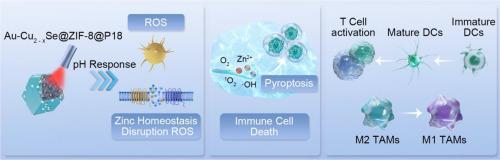
A dual-pathway pyroptosis inducer based on Au–Cu2-xSe@ZIF-8 enhances tumor immunotherapy by disrupting the zinc ion homeostasis
The regulation of intracellular ionic homeostasis to trigger antigen-specific immune responses has attracted extensive interest in tumor therapy. In this study, we developed a dual-pathway nanoreactor, Au-Cu2-xSe@ZIF-8@P18 NPs (ACS-Z-P NPs), which targets danger-associated molecular patterns (DAMPs) and releases Zn2+ and reactive oxygen species (ROS) within the tumor microenvironment (TME). Zn2+ released from the metal–organic frameworks (MOFs) was deposited in the cytoplasm, leading to aberrant transcription levels of intracellular zinc-regulated proteins and DNA damage, thereby inducing pyroptosis and immunogenic cell death (ICD) dependent on caspase1/gasdermin D (GSDMD) pathway. Furthermore, upon laser irradiation, ACS-Z-P NPs could break through the limitations of inherent defects of immunosuppression in TME, enhance ROS generation through a Fenton-like reaction cascade, which subsequently triggered the activation of inflammatory vesicles and the release of damage-associated molecular patterns (DAMPs). This cascade effect led to the amplification of pyroptosis and immunogenic cell death (ICD), thereby remodeling the immunosuppressed TME. Consequently, this process improved dendritic cell (DC) antigen presentation and augmented anti-tumor T-cell responses, effectively initiating antigen-specific immune responses and further enhancing pyroptosis and ICD. This study explores the therapeutic properties of these mechanisms in detail.
Statement of significance
The synthesized Au-Cu2-xSe@ZIF-8@P18 nanoparticles (ACS-Z-Ps) can effectively enhance the bodyʼs immune response by regulating zinc ion levels within cells. This regulation leads to abnormal levels of zinc-regulated protein transcription and DNA damage, which induces cellular pyroptosis. As a result, antigen presentation to dendritic cells (DCs) is improved, and anti-tumor T-cell responses are enhanced.
The ACS-Z-P NPs overcome the limitations of ROS deficiency and immunosuppression in the tumor microenvironment by using H2O2 in the tumor microenvironment through a Fenton-like reaction. This leads to an increased production of ROS and O2, remodeling of the immunosuppressed tumor microenvironment, and enhanced induction of cell pyroptosis and immunogenic cell death.
ACS-Z-P NPs targeted B16 cells using the photosensitizer P18 in combination with PDT treatment. This approach significantly inhibited the proliferation of B16 cells and effectively inhibited tumor growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


