金针菇中的麦角甾烷及其对癌细胞的抑制作用:体外和硅学评估。
IF 2.1
4区 医学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究从变色金针菇子实体中分离出了五种类固醇化合物。其中,9,19-环羊齿甾-3,29-二醇(3)、麦角甾-7,22-二烯-3-乙酸酯(4)和麦角甾-8(14),22-二烯-3β,5α,6β,7α-四醇(5)是首次从 Trametes versicolor 中鉴定出的化合物。对这五种化合物对癌细胞株的活性进行了评估。结果发现,化合物 5α,8α-表二氧麦角甾-6,22-二烯-3β-醇(1)对大多数受测癌细胞株最有效。硅学研究表明,化合物 1 与不同的癌症靶点,即细胞周期蛋白依赖性激酶 2(cdk2)、人类细胞周期蛋白依赖性激酶 6(cdk6)、人类 Topo IIa ATPase/AMP-PNP、抗凋亡蛋白 Bcl-2 和 Vegfr-2 具有良好的结合亲和力。根据利宾斯基的 "5 "法则和它的 ADME/Tox 特性,它也类似于药物。因此,化合物 1 是治疗癌症的理想候选药物。这些结果进一步表明,T. versicolor 是治疗癌症的潜在药物来源或药物线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
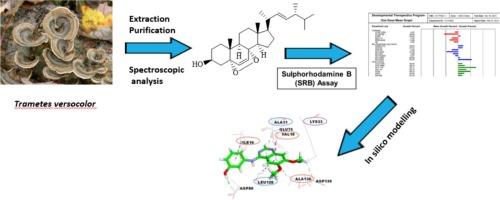
Ergostanes from the mushroom Trametes versicolor and their cancer cell inhibition: In vitro and in silico evaluation
In this study, five steroid compounds were isolated from the fruiting bodies mushroom Trametes versicolor. The compounds, 9,19-cyclolanostane-3,29-diol (3), ergosta-7,22-dien-3-acetate (4), and ergosta-8(14),22-dien-3β,5α,6β,7α-tetrol (5), were identified from T. versicolor for the first time. The five compounds were evaluated for their activity against cancer cell lines. Compound 5α,8α–epidioxyergosta-6,22-dien-3β-ol (1) was found to be the most effective against most of the cancer cell lines tested. In silico studies showed that compound 1 has good binding affinities to different cancer targets, namely cyclin-dependent kinase 2 (cdk2), human cyclin-dependent kinase 6 (cdk6), Human Topo IIa ATPase/AMP-PNP, anti-apoptotic protein Bcl-2, and Vegfr-2. It’s also druglike based on Lipinski’s rule of five and it’s ADME/Tox properties. Therefore, compound 1 is a good candidate in the management of cancer. These results further show that T. versicolor is a potential source of drugs or drug leads for cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Steroids
医学-内分泌学与代谢
CiteScore
5.10
自引率
3.70%
发文量
120
审稿时长
73 days
期刊介绍:
STEROIDS is an international research journal devoted to studies on all chemical and biological aspects of steroidal moieties. The journal focuses on both experimental and theoretical studies on the biology, chemistry, biosynthesis, metabolism, molecular biology, physiology and pharmacology of steroids and other molecules that target or regulate steroid receptors. Manuscripts presenting clinical research related to steroids, steroid drug development, comparative endocrinology of steroid hormones, investigations on the mechanism of steroid action and steroid chemistry are all appropriate for submission for peer review. STEROIDS publishes both original research and timely reviews. For details concerning the preparation of manuscripts see Instructions to Authors, which is published in each issue of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


