用于多路复用体内功能成像的模块化化学钙指示剂。
IF 36.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
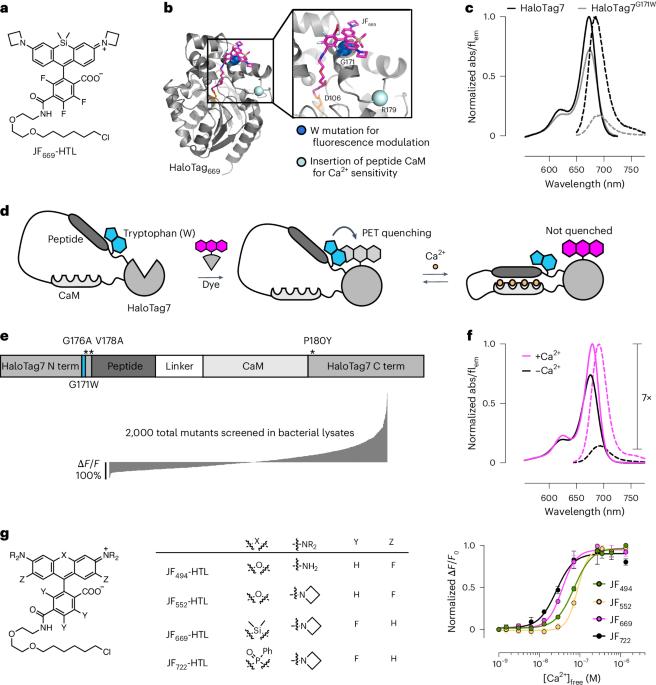
通过基因编码的荧光钙指示剂可以记录细胞分辨率的生理变化。然而,要同时对多种信号进行成像,还需要能与体内现有工具复用的明亮、基因靶向性指示剂。在这里,我们描述了 WHaloCaMP,一种由明亮染料配体和蛋白质传感器结构域组成的模块化化学基因钙指示剂。WHaloCaMP 中的荧光变化来自于通过策略性放置的色氨酸对结合染料的可逆淬灭。WHaloCaMP 与罗丹明染料配体兼容,这些染料配体可发出从绿色到近红外的荧光,其中包括几种可有效标记动物大脑的染料配体。当与近红外染料配体结合时,WHaloCaMP的荧光强度增加7倍,钙结合后的荧光寿命增加2.1-ns。我们利用 WHaloCaMP1a 对苍蝇和小鼠体内的 Ca2+ 反应进行成像,对斑马鱼幼体中的数百个神经元和星形胶质细胞进行三色多重功能成像,并利用荧光寿命成像显微镜(FLIM)对 Ca2+ 浓度进行量化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A modular chemigenetic calcium indicator for multiplexed in vivo functional imaging
Genetically encoded fluorescent calcium indicators allow cellular-resolution recording of physiology. However, bright, genetically targetable indicators that can be multiplexed with existing tools in vivo are needed for simultaneous imaging of multiple signals. Here we describe WHaloCaMP, a modular chemigenetic calcium indicator built from bright dye-ligands and protein sensor domains. Fluorescence change in WHaloCaMP results from reversible quenching of the bound dye via a strategically placed tryptophan. WHaloCaMP is compatible with rhodamine dye-ligands that fluoresce from green to near-infrared, including several that efficiently label the brain in animals. When bound to a near-infrared dye-ligand, WHaloCaMP shows a 7× increase in fluorescence intensity and a 2.1-ns increase in fluorescence lifetime upon calcium binding. We use WHaloCaMP1a to image Ca2+ responses in vivo in flies and mice, to perform three-color multiplexed functional imaging of hundreds of neurons and astrocytes in zebrafish larvae and to quantify Ca2+ concentration using fluorescence lifetime imaging microscopy (FLIM). WHaloCaMP is a chemigenetic calcium indicator that can be combined with different rhodamine dyes for multiplexed or FLIM imaging in vivo, as demonstrated for calcium imaging in neuronal cultures, brain slices, Drosophila, zebrafish larvae and the mouse brain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


