药片随时间变化的松弛试验:修订解释。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
松弛试验通常用于制药领域,以评估药粉和药片的应变速率敏感性。这些测试包括对模具中的粉末施加恒定应变,然后监测应力随时间的变化。解释这些测试非常复杂,因为同时会出现不同的物理现象,主要是粘弹性和粘塑性。仅观察轴向压力的变化无法区分这两种现象,因为在这两种情况下,轴向压力都会下降。这项研究表明,监测松弛过程中模壁压力的变化有助于区分这两种现象的影响。理论分析表明,在粘弹性过程中,模壁压力也会降低,而在松弛过程中,模壁压力的增加则表明发生了粘弹性松弛。使用专门设计的压实循环对四种不同的药用辅料进行实验证实了这一点。实验结果表明,在低压下,粘弹性占主导地位,而在高压下,粘弹性变得更加突出。这些结果表明,在低压条件下,松弛试验可用于评估不同产品的粘塑性。不过,应始终避免使用高压,因为粘弹性现象可能会变得更加显著,而这两种现象的结合可能会影响解释结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
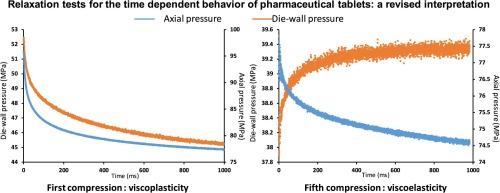
Relaxation tests for the time dependent behavior of pharmaceutical tablets: A revised interpretation
Relaxation tests are often used in the pharmaceutical field to assess the strain rate sensitivity of pharmaceutical powders and tablets. These tests involve applying a constant strain to the powder in the die and then monitoring the stress evolution over time. Interpreting these tests is complicated because different physical phenomena, mainly viscoelasticity and viscoplasticity, occur simultaneously. These two phenomena cannot be distinguished by observing the evolution of the axial pressure alone, as it decreases in both cases. In this work, it was shown that monitoring the evolution of the die-wall pressure during relaxation can help separate the effects of these phenomena. Theoretical considerations revealed that during viscoplasticity, the die-wall pressure also decreases, whereas an increase in the die-wall pressure during relaxation indicates a viscoelastic relaxation. This was confirmed experimentally using specially designed compaction cycles on four different pharmaceutical excipients. Experimental results indicated that at low pressure, viscoplasticity was predominant, whereas at high pressure, viscoelasticity became more prominent. These results suggest that at low pressures, relaxation tests can be used to assess the viscoplastic properties of different products. However, the use of high pressure should always be avoided as viscoelastic phenomena might become more significant, and the combination of both phenomena might compromise the interpretation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


