卵泡刺激素会促进心房颤动绝经妇女的心房纤维化。
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
背景:与男性相比,绝经后女性房颤患者的心房纤维化程度更高,消融术后的复发率也更高。然而,其潜在机制仍不清楚:目的:研究更年期促进心房纤维化的机制:方法:在一个前瞻性房颤女性队列中进行回归分析,以评估低电压区(LVA)与性激素水平之间的关系。自发性房颤模型 CREM-IbΔC-X 小鼠接受了双侧卵巢切除术(OVX)。进行了心电图、超声心动图和马森染色。雄性小鼠接受 FSH 刺激三个月。在进行程序电刺激和结构分析后,还对血管紧张素 II(Ang II)诱导的压力超负荷小鼠模型进行了 OVX。为了阐明潜在的机制,还进行了大量 RNA 测序(RNA-seq):结果:女性的 LVA 负荷明显高于男性(PC 结论:绝经后女性的 LVA 负荷明显高于男性:绝经后女性的 LVA 负担高于男性,且与 FSH 水平呈正相关。FSH通过氧化应激促进心房纤维化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
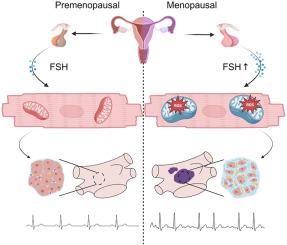
Follicle-stimulating hormone promotes atrial fibrosis in menopausal women with atrial fibrillation
Background
Postmenopausal women with atrial fibrillation (AF) exhibit a higher level of atrial fibrosis and a higher recurrence rate after ablation compared with men. However, the underlying mechanism remains unclear.
Objective
The purpost of this study was to investigate the mechanism through which menopause promotes atrial fibrosis.
Methods
In a prospective cohort of women with AF, regression analyses were conducted to assess the relationship between low-voltage area (LVA) and sex hormone levels. CREM-IbΔC-X mice, a spontaneous AF model, underwent bilateral ovariectomy (OVX). Electrocardiograms, echocardiograms, and Masson staining were performed. Follicle-stimulating hormone (FSH) stimulation was applied in male mice for 3 months. OVX was also applied in an angiotensin II (Ang II)–induced pressure overload mouse model, after programmed electrical stimulation and structural analyses. Bulk RNA sequencing (RNA-seq) was performed to elucidate potential mechanisms.
Results
Women demonstrated a significantly higher LVA burden than men (P < .001). A positive correlation was observed between LVA burden and FSH level (P = .002). Mice in the OVX group exhibited a significantly higher incidence of AF (P = .040) and atrial fibrosis (P = .021) compared with the Sham group, which could be attenuated by adeno-associated virus encoding small interfering RNA against Fshr. In male CREM-IbΔC-X mice, FSH stimulation promoted the occurrence of AF (P = .035) and atrial fibrosis (P = .002). In Ang II–induced female mice, OVX prompted atrial fibrosis, increased AF inducibility, and shortened atrial effective refractory period, which could be attenuated with knockdown of Fshr. RNA-seq indicated mitochondrial dysfunction.
Conclusion
Postmenopausal women exhibited a higher LVA burden than men, which was positively correlated with FSH level. FSH promoted atrial fibrosis through oxidative stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Heart rhythm
医学-心血管系统
CiteScore
10.50
自引率
5.50%
发文量
1465
审稿时长
24 days
期刊介绍:
HeartRhythm, the official Journal of the Heart Rhythm Society and the Cardiac Electrophysiology Society, is a unique journal for fundamental discovery and clinical applicability.
HeartRhythm integrates the entire cardiac electrophysiology (EP) community from basic and clinical academic researchers, private practitioners, engineers, allied professionals, industry, and trainees, all of whom are vital and interdependent members of our EP community.
The Heart Rhythm Society is the international leader in science, education, and advocacy for cardiac arrhythmia professionals and patients, and the primary information resource on heart rhythm disorders. Its mission is to improve the care of patients by promoting research, education, and optimal health care policies and standards.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


