低针叶脑内皮细胞主要利用膜融合来内化细胞外囊泡。
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-09-19
DOI:10.1016/j.ejpb.2024.114500
引用次数: 0
摘要
细胞外囊泡(EVs)是一类新兴的药物载体,据报道主要通过多种内吞途径内化到受体细胞,如凝胶酶介导、洞穴介导和大蛋白胞吞途径。在这项工作中,(1) 我们通过研究同源 EV(EV 供体细胞和同种类型的受体细胞)与异源 EV(EV 供体细胞和不同类型的受体细胞)的细胞摄取,调查了同型与异型相互作用的潜在影响;(2) 确定了 EV 内化到低针胞/难于递送细胞模型(如脑内皮细胞(BECs)和吞噬细胞模型(如巨噬细胞)的途径。与异型相互作用相比,同型相互作用导致受体 BECs 更大程度的吸收。然而,当使用药理抑制剂阻断内吞途径时,我们并没有看到受体BECs对EV的摄取完全减少,我们基于R18融合试验的研究结果表明,EV主要通过膜融合而不是依靠内吞作用进入低刺吸细胞受体BECs。亲脂性 PKH67 染料标记的 EV 而非静脉内酯酶激活的钙黄绿素酯标记的 EV 严重降低了 BECs 对颗粒的摄取,而吞噬型巨噬细胞内化这两种 EV 标记颗粒的程度相当。我们的研究结果还强调了仔细选择标记染料化学成分来研究EV摄取的重要性,尤其是对于像BECs这样的低针状细胞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
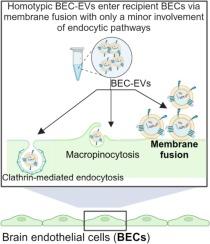
Low pinocytic brain endothelial cells primarily utilize membrane fusion to internalize extracellular vesicles
Extracellular vesicles (EVs) are an emerging class of drug carriers and are primarily reported to be internalized into recipient cells via a combination of endocytic routes such as clathrin-mediated, caveolae-mediated and macropinocytosis pathways. In this work, (1) we investigated potential effects of homotypic vs. heterotypic interactions by studying the cellular uptake of homologous EVs (EV donor cells and recipient cells of the same type) vs. heterologous EVs (EV donor cells and recipient cells of different types) and (2) determined the route of EV internalization into low pinocytic/hard-to-deliver cell models such as brain endothelial cells (BECs). Homotypic interactions led to a greater extent of uptake into the recipient BECs compared to heterotypic interactions. However, we did not see a complete reduction in EV uptake into recipient BECs when endocytic pathways were blocked using pharmacological inhibitors and our findings from a R18-based fusion assay suggest that EVs primarily use membrane fusion to enter low-pinocytic recipient BECs instead of relying on endocytosis. Lipophilic PKH67 dye-labeled EVs but not intravesicular esterase-activated calcein ester-labeled EVs severely reduced particle uptake into BECs while phagocytic macrophages internalized EVs labeled with both dyes to comparable extents. Our results also highlight the importance of carefully choosing labeling dye chemistry to study EV uptake, especially in the case of low pinocytic cells such as BECs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


