比较可电离脂质在脂质纳米粒子介导的 DNA 传输中的应用。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
脂质纳米颗粒(LNPs)已成功用于基于 RNA 的基因递送。然而,在基因替代疗法中,使用 LNPs 作为非病毒载体递送 DNA 表达质粒可能是诱导更持久疗效的一种有前途的策略。因此,我们将编码荧光标记或荧光素酶的 DNA 表达质粒(3 至 4 kbp)与 LNPs 结合在一起。测试了临床上使用的不同可电离脂质(DLin-MC3-DMA、SM-102 和 ALC-0315),以比较它们对 DNA 质粒递送的影响。对 DNA-LNPs 的胶体特性(大小、多分散性、ζ电位、形态)、体外性能(细胞摄取、DNA 递送和基因表达)和体内特征(生物分布和荧光素酶基因表达)进行了表征。在 N/P 比为 6 的优化条件下,获得了具有阴离子ζ电位的球形、小而单分散的颗粒。每个初始培养的细胞只需最少 1 pg DNA,就能实现高效的转基因表达。通过对斑马鱼的研究,可以筛选出DNA-LNPs,这些DNA-LNPs能延长血液循环,避免巨噬细胞清除和血管外渗。我们的比较研究表明,可电离脂质类型对 DNA-LNP 的性能影响很大。在野生型小鼠体内,含有可电离脂质 ALC-0315 的 DNA-LNPs 的转染效率更高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
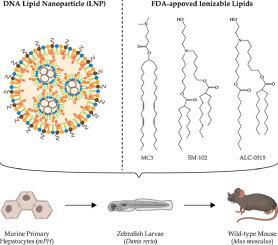
Comparison of ionizable lipids for lipid nanoparticle mediated DNA delivery
Lipid nanoparticles (LNPs) are successfully used for RNA-based gene delivery. In the context of gene replacement therapies, however, delivery of DNA expression plasmids using LNPs as a non-viral vector could be a promising strategy for the induction of longer-lasting effects.
Therefore, DNA expression plasmids (3 to 4 kbp) coding for fluorescent markers or luciferase were combined with LNPs. Different clinically used ionizable lipids (DLin-MC3-DMA, SM-102, and ALC-0315) were tested to compare their influence on DNA plasmid delivery.
DNA-LNPs were characterized with respect to their colloidal properties (size, polydispersity, ζ-potential, morphology), in vitro performance (cellular uptake, DNA delivery, and gene expression), and in vivo characteristics (biodistribution and luciferase gene expression). At an optimized N/P ratio of 6, spherical, small and monodisperse particles with anionic ζ-potential were obtained. Efficient transgene expression was achieved with a minimum amount of 1 pg DNA per initially plated cells. Zebrafish studies allowed selection of DNA-LNPs, which demonstrated prolonged blood circulation, avoidance of macrophage clearance, and vascular extravasation.
Our comparative study demonstrates a high impact of the ionizable lipid type on DNA-LNP performance. Superior transfection efficiency of DNA-LNPs containing the ionizable lipid ALC-0315 was confirmed in wildtype mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


