Shh 可调节缺血性中风后 M2 小胶质细胞的极化和纤维化瘢痕的形成。
IF 4.4
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:纤维化瘢痕的形成是影响缺血性中风后组织重建和功能恢复的关键病理变化。中枢神经系统(CNS)纤维化瘢痕背后的调控机制在很大程度上仍不为人所知。虽然已知巨噬细胞在外周组织的纤维化瘢痕形成中发挥作用,但中枢神经系统的常驻免疫细胞小胶质细胞参与中枢神经系统纤维化的情况还需要进一步探索。音速刺猬(Shh)信号通路在胚胎发育和组织再生中起着关键作用,在调节外周组织纤维化方面也至关重要。然而,Shh 对缺血性脑卒中后纤维化瘢痕形成的影响和调控机制尚未得到深入研究:方法:本研究通过在体内和体外操纵 Shh 的表达,探讨 Shh 能否调控缺血性脑卒中后纤维化瘢痕的形成及其内在机制:结果:我们的研究结果表明,急性缺血性脑卒中患者的血清以及 MCAO/R 小鼠的血清、脑脊液和缺血区域中 Shh 表达上调。此外,Shh表达的上调与纤维化瘢痕形成和M2小胶质细胞极化呈正相关。敲除Shh可抑制纤维化瘢痕形成和M2小胶质细胞极化,同时加重MCAO/R小鼠的神经功能缺损。在体外,腺病毒敲除或平滑激动剂(SAG)激活OGD/R后BV2细胞中的Shh表达,可调节其极化,并影响TGFβ1和PDGFA的表达,进而影响成纤维细胞的活化:这些结果表明,Shh 可调控脑缺血后 M2 小胶质细胞的极化和纤维化瘢痕的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
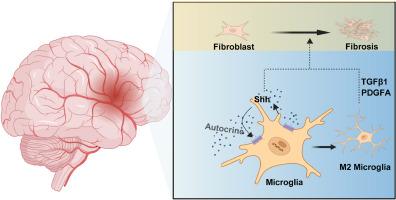
Shh regulates M2 microglial polarization and fibrotic scar formation after ischemic stroke
Background
Fibrotic scar formation is a critical pathological change impacting tissue reconstruction and functional recovery after ischemic stroke. The regulatory mechanisms behind fibrotic scarring in the central nervous system (CNS) remain largely unknown. While macrophages are known to play a role in fibrotic scar formation in peripheral tissues, the involvement of microglia, the resident immune cells of the CNS, in CNS fibrosis requires further exploration. The Sonic Hedgehog (Shh) signaling pathway, pivotal in embryonic development and tissue regeneration, is also crucial in modulating fibrosis in peripheral tissues. However, the impact and regulatory mechanisms of Shh on fibrotic scar formation post-ischemic stroke have not been thoroughly investigated.
Methods
This study explores whether Shh can regulate fibrotic scar formation post-ischemic stroke and its underlying mechanisms through in vivo and in vitro manipulation of Shh expression.
Results
Our results showed that Shh expression was upregulated in the serum of acute ischemic stroke patients, as well as in the serum, CSF, and ischemic regions of MCAO/R mice. Moreover, the upregulation of Shh expression was positively correlated with fibrotic scar formation and M2 microglial polarization. Shh knockdown inhibited fibrotic scar formation and M2 microglial polarization while aggravating neurological deficits in MCAO/R mice. In vitro, adenoviral knockdown or Smoothened Agonist (SAG) activation of Shh expression in BV2 cells following OGD/R regulated their polarization and influenced the expression of TGFβ1 and PDGFA, subsequently affecting fibroblast activation.
Conclusion
These results suggest that Shh regulates M2 microglial polarization and fibrotic scar formation after cerebral ischemia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurochemistry international
医学-神经科学
CiteScore
8.40
自引率
2.40%
发文量
128
审稿时长
37 days
期刊介绍:
Neurochemistry International is devoted to the rapid publication of outstanding original articles and timely reviews in neurochemistry. Manuscripts on a broad range of topics will be considered, including molecular and cellular neurochemistry, neuropharmacology and genetic aspects of CNS function, neuroimmunology, metabolism as well as the neurochemistry of neurological and psychiatric disorders of the CNS.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


