微生理系统中的肝毒性评估:模拟过量对乙酰氨基酚在肠肝芯片上的吸收和毒性过程。
IF 3.9
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
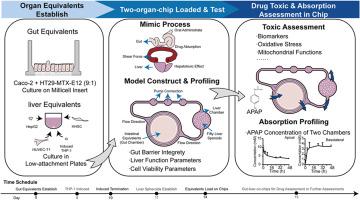
为了弥补动物模型的局限性,人们提出了新的药物安全性评价模型,以完善和减少现有模型。为了在体外肠肝微生理系统(MPS)中模拟药物的吸收和代谢,预测毒代动力学和毒性效应,我们构建了肠肝芯片,并检测了过量对乙酰氨基酚(APAP)后的急性肝损伤过程。我们利用 Caco-2 和 HT29-MTX-E12 细胞系建立肠道等效细胞系,并利用 PMA 诱导的 HepG2、HUVEC-T1 和 THP-1 以及人肝星状细胞建立肝脏等效细胞系。采用高效液相色谱法测定了 APAP 的浓度,并采用 Phoenix 非室分析法拟合了毒代动力学参数。肝损伤生物标志物天冬氨酸氨基转移酶和丙氨酸氨基转移酶以及肝功能标志物白蛋白的变化表明,芯片上两个器官模型的短期培养在 4 天内是稳定的。服用 APAP 后,活性氧信号转导增强,线粒体膜电位降低,caspase-3 被激活,p53 信号转导增强,这表明 APAP 过量会诱发毒性反应。在肠道-肝脏 MPS 模型中,我们拟合了毒物动力学参数,模拟了过量服用 APAP 后的肝毒性过程,这将促进芯片上器官在药物毒性检测中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hepatotoxic assessment in a microphysiological system: Simulation of the drug absorption and toxic process after an overdosed acetaminophen on intestinal-liver-on-chip
To compensate the limitation of animal models, new models were proposed for drug safety evaluation to refine and reduce existing models. To mimic drug absorption and metabolism and predict toxicokinetic and toxic effects in an in vitro intestinal-liver microphysiological system (MPS), we constructed an intestinal-liver-on-chip and detected the acute liver injury process after an overdose of acetaminophen (APAP). Caco-2 and HT29-MTX-E12 cell lines were utilized to establish intestinal equivalents, along with HepG2, HUVEC-T1, and THP-1 induced by PMA and human hepatic stellate cell to establish liver equivalents. The APAP concentration was determined using high-performance liquid chromatography, and the toxicokinetic parameters were fitted using the non-compartmental analysis method by Phoenix. Changes in liver injury biomarkers aspartate aminotransferase and alanine aminotransferase, and liver function marker albumin indicated that the short-term culture of the two organs-on-chip model was stable for 4 days. Reactive oxygen species signaling was enhanced after APAP administration, along with decreased mitochondrial membrane potential, activated caspase-3, and enhanced p53 signaling, indicating a toxic response induced by APAP overdose. In the gut-liver MPS model, we fitted the toxicokinetic parameters and simulated the hepatotoxicity procedure following an APAP overdose, which will facilitate the organ-on-chips application in drug toxicity assays.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


