全面了解生物仿生全合成布雷维纳酰胺 A 的机理。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
最近,有关化学合成布雷维那酰胺 A(BA)的几项研究得到了报道。其中,Lawrence 的研究小组报道了一种高效且具有显著选择性的合成策略。然而,人们对这些结果仍缺乏统一的机理认识。我们开展了一项 DFT 研究,并提出了一种统一的机理来理解这些实验结果。从中间体 2 开始,最有利的反应顺序是快速同分异构,然后是碱基分子的 σ 迁移,最后是反电子需求的 Diels-Alder 反应,从而立体选择性地形成 BA 产物。这一反应机理也可用于理解涉及酶催化的 BA 生物合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
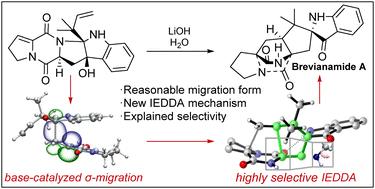
A comprehensive understanding of the mechanism of the biomimetic total synthesis of brevianamide A†
Recently, several studies on the chemical synthesis of brevianamide A (BA) were reported. In particular, a highly efficient and remarkably selective synthetic strategy was reported by Lawrence's group. However, a unified mechanistic understanding of these results is still lacking. We have carried out a DFT study and proposed a unified mechanism to understand these experimental results. Starting from intermediate 2, the most favorable reaction sequence is a fast tautomerization, followed by a σ-migration of the base moiety, and a final inverse-electron demanding Diels–Alder reaction, resulting in the formation of the BA product stereoselectively. This reaction mechanism can also be applied to understand the biosynthesis of BA that involves enzymatic catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


