靛红和 2-氯吡啶鎓盐的反应:α、β-不饱和羰基吲哚的高效和非对映选择性合成。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究开发了一种新的、温和的非对映选择性方法,通过异汀和 2-氯吡啶鎓盐在 EtOH 中于室温下反应 5 分钟,合成 β-吡啶酮-α,β-不饱和吲哚。该方法反应条件温和,产物收率高且非对映选择性好,对各种官能团具有良好的耐受性。主要的异构体是 Z 异构体,在 NaN3 存在下可转化为 E 异构体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
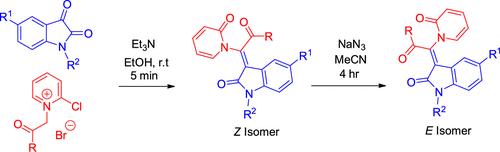
Reaction of Isatin and 2-Chloropyridinium Salt: An Efficient and Diastereoselective Synthesis of α,β-Unsaturated Oxindoles.
A new, mild, and diastereoselective method has been developed for the synthesis of β-pyridone-α,β-unsaturated oxindoles by the reaction of isatins and 2-chloropyridinium salts in EtOH at room temperature for 5 min. This method operates under mild reaction conditions, providing the product with a good yield and diastereoselectivity, and it exhibits excellent tolerance toward various functional groups. The predominant isomer is the Z isomer, which can convert to the E isomer in the presence of NaN3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


