阳离子对 Cu 上电化学 CO 还原反应基本步骤的影响
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电解质中阳离子的性质对二氧化碳和一氧化碳电化学还原反应(CO(2)RR)的性能有重大影响,但其分子水平上的机理仍存在争议。我们对阳离子如何影响电化学界面上的关键物理化学变量以及 CO(2)RR 的基本步骤的理解还存在很大差距。在这项工作中,我们定量测定了阳离子对电化学条件下 CO 在铜上吸附的焓和熵的影响。在 Li+ > Na+ > K+ > Cs+ 的序列中,CO 的吸附变得越来越不利,并产生了很大的焓熵补偿效应。重要的是,阳离子以相反的方向影响 CORR 初始状态和过渡状态的稳定性。我们的研究结果让我们深入了解了阳离子对 CORR 中各个基本步骤的影响,并证明了在吸附 CO 的转化过程中稳定过渡态的能力是一个决定性因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
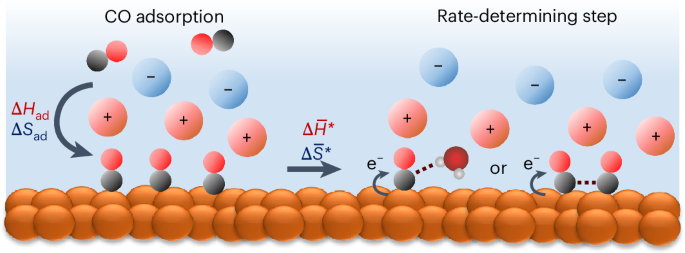
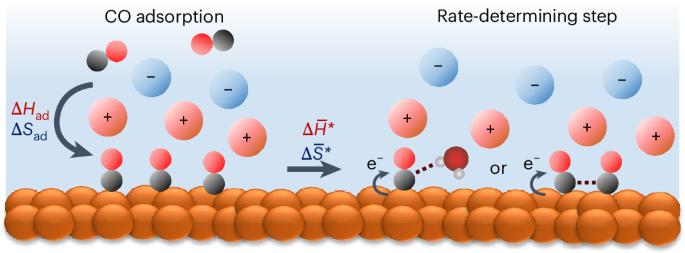
Cation effect on the elementary steps of the electrochemical CO reduction reaction on Cu
The nature of the cations in an electrolyte has a substantial impact on the performance of the electrochemical CO2 and CO reduction reaction (CO(2)RR), however, its mechanism at the molecular level remains the subject of debate. Major gaps in our understanding include how cations affect key physicochemical variables at electrochemical interfaces and the elementary steps of the CO(2)RR. In this work, we have quantitatively determined the impact of cations on the enthalpy and entropy of CO adsorption on Cu under electrochemical conditions. CO adsorption becomes increasingly unfavourable in the sequence Li+ > Na+ > K+ > Cs+ with a substantial enthalpy–entropy compensation effect. Importantly, cations affect the stability of the initial and transition states of the CORR in opposite directions. Our results provide insights into the effect of cations on individual elementary steps in the CORR and demonstrate that the ability to stabilize the transition state in the conversion of adsorbed CO is a decisive factor. The mechanism of electrocatalytic CO/CO2 reduction on Cu surfaces is complex and its various mechanisms remain under debate, including the important role of cations in the electrolyte. Here the authors quantitatively determine the impact of alkali cations on the thermodynamics of CO adsorption under electrochemical conditions and the activation parameters of the rate-determining step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


