开发基于可调机制的碳糖配体,通过形成瞬时共价中间体稳定糖苷水解酶
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
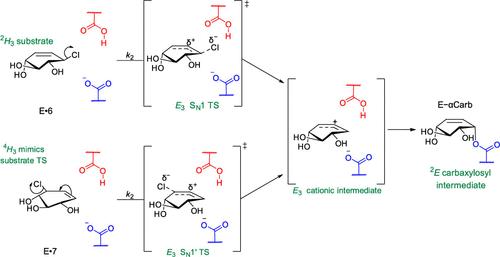
人类溶酶体糖苷水解酶中的许多成员发生突变,会导致多种溶酶体贮积疾病。因此,人们一直在努力寻找这些溶酶体酶的药理伴侣。大多数候选合剂都是活性位点定向竞争性亚氨基糖抑制剂,但成功率有限。作为向另类药理伴侣迈出的第一步,我们探索了基于机制的小分子可逆共价抑制剂形成瞬时酶抑制剂加合物的潜力。通过对候选分子进行系列合成和动力学分析,我们发现,合理调整葡萄糖构型碳糖的化学反应活性,可以得到环己烯基烯丙基碳糖,它们能与β-葡糖脑酶(GCase)溶酶体酶发生反应,形成具有不同半衰期的共价酶加合物。对这些与 GCase 非共价结合的化合物进行的 X 射线结构分析,以及与 GCase 催化亲核体反应的化合物共价加合物的结构分析,揭示了这些化合物意想不到的反应活性。利用差示扫描荧光测定法,我们发现瞬时共价中间体的形成可以稳定折叠酶,使其不受热变性的影响。此外,这些共价加合物分解后会释放出活性酶和一种不再具有抑制作用的产物。我们进一步发现,有一种化合物通过一种前所未有的类似 SN1′的机理发生反应,表现出特殊的反应活性--这种化合物还能共价标记α-葡萄糖苷酶就是证明。我们预计,这种基于碳糖的单一周转共价配体可作为溶酶体糖苷水解酶和其他疾病相关的保留糖苷酶的药理学伴侣。这些分子不寻常的反应性也为创造新的化学生物学探针打开了大门,以探索这一重要的糖苷水解酶超家族的生物学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development of Tunable Mechanism-Based Carbasugar Ligands that Stabilize Glycoside Hydrolases through the Formation of Transient Covalent Intermediates
Mutations in many members of the set of human lysosomal glycoside hydrolases cause a wide range of lysosomal storage diseases. As a result, much effort has been directed toward identifying pharmacological chaperones of these lysosomal enzymes. The majority of the candidate chaperones are active site-directed competitive iminosugar inhibitors but these have met with limited success. As a first step toward an alternative class of pharmacological chaperones we explored the potential of small molecule mechanism-based reversible covalent inhibitors to form transient enzyme–inhibitor adducts. By serial synthesis and kinetic analysis of candidate molecules, we show that rational tuning of the chemical reactivity of glucose-configured carbasugars delivers cyclohexenyl-based allylic carbasugar that react with the lysosomal enzyme β-glucocerebrosidase (GCase) to form covalent enzyme-adducts with different half-lives. X-ray structural analysis of these compounds bound noncovalently to GCase, along with the structures of the covalent adducts of compounds that reacted with the catalytic nucleophile of GCase, reveal unexpected reactivities of these compounds. Using differential scanning fluorimetry, we show that formation of a transient covalent intermediate stabilizes the folded enzyme against thermal denaturation. In addition, these covalent adducts break down to liberate the active enzyme and a product that is no longer inhibitory. We further show that the one compound, which reacts through an unprecedented SN1′-like mechanism, exhibits exceptional reactivity–illustrated by this compound also covalently labeling an α-glucosidase. We anticipate that such carbasugar-based single turnover covalent ligands may serve as pharmacological chaperones for lysosomal glycoside hydrolases and other disease-associated retaining glycosidases. The unusual reactivity of these molecules should also open the door to creation of new chemical biology probes to explore the biology of this important superfamily of glycoside hydrolases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


