用于水中硫化物光氧化的供体-受体型多孔有机聚合物的分子结构工程:一种可持续的方法
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
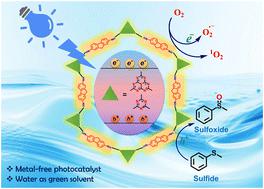
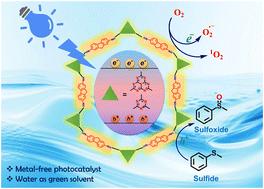
利用温和、环境友好的反应条件将硫化物光催化氧化成高价值的硫氧化物是一种可持续的方法,这种方法非常可取,但也相当具有挑战性。在此,我们提出了一种新方法,通过基于供体-受体(D-A)聚合物网络的分子结构工程来增强硫化物在水中的光催化氧化。通过加入缺电子的庚嗪或三嗪单元作为受体,富电子的 2,5 二甲基芴作为供体,实现了协同效应,促进了光吸收时电荷的有效分离。在蓝光照射下,HEP-FL 和 TZ-FL 两种聚合物网络分别在 1.3 小时和 3.5 小时内高效地将硫化物选择性氧化为亚砜,转化率达到 100%。通过先进的光谱和电化学测量,阐明了分子结构与光电特性之间的相关性,揭示了可调带状结构和激子结合能。值得注意的是,含有庚嗪的聚合物网络(HEP-FL)表现出卓越的电荷分离效率和更强的催化活性,这归因于电子析出的改善和激子结合能的降低。此外,我们还利用 HEP-FL 作为光催化剂,对氧化亚砜的合成进行了绿色指标计算,以证明反应系统的可持续性。这些发现强调了以供体-受体为基础的聚合物网络作为选择性氧化反应的高效光催化剂的重要前景,凸显了它们在有机合成和工业应用中推进环保意识的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular structural engineering of donor–acceptor-based porous organic polymers for sulfide photooxidation in water: a sustainable approach†
The utilization of mild and environment-friendly reaction conditions for the photocatalytic oxidation of sulfides to highly valuable sulfoxides represents a sustainable approach that is highly desirable yet quite challenging. Herein, we present a novel approach to enhance the photocatalytic oxidation of sulfides in water by molecular structural engineering of donor–acceptor (D–A) based polymeric networks. By incorporating electron-deficient heptazine or triazine units as acceptors and electron-rich 2,5-diamino fluorene as donors, a synergistic effect is achieved, promoting efficient charge separation upon light absorption. The two polymeric networks namely HEP-FL and TZ-FL efficiently carried out selective oxidation of sulfides to sulfoxide with 100% conversion within 1.3 h and 3.5 h, respectively under blue light irradiation. Through advanced spectroscopic and electrochemical measurements, the correlation between molecular structures and optoelectronic properties is elucidated, unveiling tunable band structures and exciton binding energies. Notably, the heptazine-containing polymeric network (HEP-FL) exhibited superior charge separation efficiency and enhanced catalytic activity, attributed to improved electron delocalization and reduced exciton binding energy. Additionally, we have performed green metrics calculations for the synthesis of sulfoxide using HEP-FL as a photocatalyst to prove the sustainability of the reaction system. These findings underscore the significant prospects of donor–acceptor-based polymeric networks as highly effective photocatalysts for selective oxidation reactions, highlighting their potential to advance environmentally conscious practices in organic synthesis and industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


