协同约束和电子催化促进 C70F70 纳米反应器中的 N2 加氢反应:理论研究
IF 5.3
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
由于 N2 极其稳定的三键和对称性禁止直接加氢,因此通过单电子激活还原或氢化 N2 异常困难。本文展示的是全氟碳笼 C70F70 中 N2 的还原或加氢反应,这是一种具有协同限制和电子催化效应的前景广阔的纳米反应器。C70F70 的所有双极性 C-F 单元都指向其中心,这不仅能捕获反应物,还能通过降低轨道能使笼子及其客体具有很强的电子托管/结合能力。这种束缚效应可迫使一个电子进入 N2 π* 轨道,不仅激活了 N≡N 键,还使其前沿分子轨道与 H2 对称匹配,从而促进其氢化反应,明显降低了能垒。通过吸收电子进入笼式反应器而启动还原反应的能力,无需表面吸附和固体催化剂,从而实现了传统电/光化学过程无法实现的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
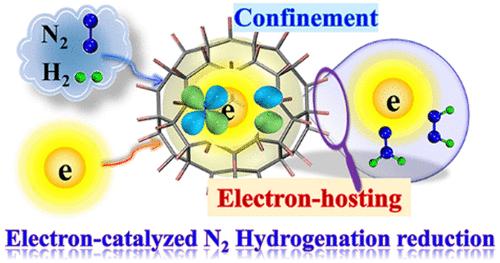
Synergetic Confinement and Electron-Catalysis Boost N2 Hydrogenation in C70F70 Nanoreactor: A Theoretical Investigation
Reduction by one-electron activation or hydrogenation of N2 is extraordinarily difficult because of its extremely stable triple bond and symmetry-forbidden direct H2 addition. Demonstrated herein is reduction or hydrogenation of N2 in perfluorocarbon cage C70F70, a promising nanoreactor with synergetic confinement and electron-catalyzing effects. C70F70 pointing all dipolar C–F units toward its center not only traps reactants but also gives the cage and its guest species strong electron-hosting/binding abilities through lowering their orbital energies. Such a confinement effect can force one electron into the N2 π*-orbital, not only activating the N≡N bond but also making its frontier molecular orbitals symmetry matching with H2 and thus boosting its hydrogenation with a noticeable lowering of the energy barrier. The ability to initiate reduction reactions by absorbing electrons into the cage-reactor without surface adsorption and solid catalysts enables pathways that are not accessible using conventional electro-/photochemical processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Nano Materials
Multiple-
CiteScore
8.30
自引率
3.40%
发文量
1601
期刊介绍:
ACS Applied Nano Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics and biology relevant to applications of nanomaterials. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


