二氯甲烷 + 硫酸烷基离子液体体系的气液平衡测量
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
离子液体 (IL) 在处理含二氯甲烷 (DCM) 的废气方面显示出巨大潜力,但目前有关 DCM + IL 系统的汽液平衡 (VLE) 数据非常有限。本研究使用顶空气相色谱 (HS-GC) 测定了 DCM + [Epy][EtSO4] 和 DCM + [Emim][EtSO4] 体系在 308.15 K、313.15 K、318.15 K、323.15 K 和 328.15 K 下的等温 VLE 数据。结果表明,DCM + [Epy][EtSO4] 和 DCM + [Emim][EtSO4] 系统与 NRTL 模型的拟合平均相对偏差 (ARD) 分别为 6.97% 和 7.95%,与 UNIQUAC 模型的拟合 ARD 分别为 6.10% 和 6.28%。两个系统的 UNIQUAC 模型的拟合效果均优于 NRTL 模型。在这些模型下,计算得出的 DCM 活性系数均小于 1,表明溶液与 Raoult 定律呈负偏差,且溶液的非理想性随 IL 浓度的增加而增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
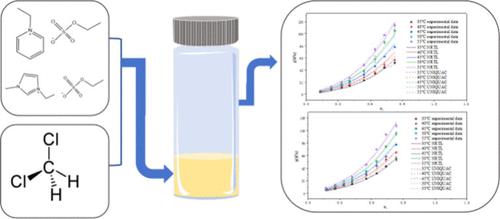
Measurement of Vapor–Liquid Equilibrium for Dichloromethane + Alkyl Sulfate-Based Ionic Liquid Systems
Ionic liquids (ILs) show great potential for the treatment of dichloromethane (DCM)-containing waste gases, but currently there are limited data on the vapor–liquid equilibrium (VLE) of DCM + IL systems. This work uses headspace gas chromatography (HS-GC) to determine the isothermal VLE data of the DCM + [Epy][EtSO4] and DCM + [Emim][EtSO4] systems at 308.15 K, 313.15 K, 318.15 K, 323.15 K, and 328.15 K. The nonrandom two-liquid (NRTL) and universal quasi-chemical correlation activity coefficient (UNIQUAC) models are used to regress the VLE data. The results show that the fitting average relative deviations (ARDs) of the DCM + [Epy][EtSO4] and DCM + [Emim][EtSO4] systems to the NRTL model were 6.97% and 7.95% and the fitting ARDs to the UNIQUAC model were 6.10% and 6.28%, respectively. The fitting effects of the UNIQUAC models for both systems were better than those of the NRTL model. With the models, the calculated activity coefficients of DCM were all less than 1, indicating that the solutions exhibited negative deviations from Raoult’s law, and the nonideality of the solutions increased with increasing IL concentration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


